

Connecting Innovation to Purpose NASDAQ: CRBP Corporate Presentation February 14, 2025 Exhibit 99.2

This presentation contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and Private Securities Litigation Reform Act, as amended, including those relating to the Company’s trial results, product development, clinical and regulatory timelines, market opportunity, competitive position, possible or assumed future results of operations, business strategies, potential growth opportunities, including timing or completion of trials and presentation of data and other statements that are predictive in nature. These forward-looking statements are based on current expectations, estimates, forecasts and projections about the industry and markets in which we operate and management’s current beliefs and assumptions. These statements may be identified by the use of forward-looking expressions, including, but not limited to, “expect,” “anticipate,” “intend,” “plan,” “believe,” “estimate,” “potential,” “predict,” “project,” “should,” “would” and similar expressions and the negatives of those terms. These statements relate to future events or our financial performance and involve known and unknown risks, uncertainties, and other factors, on our operations, clinical development plans and timelines, which may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Such factors include those set forth in the Company’s filings with the Securities and Exchange Commission. Prospective investors are cautioned not to place undue reliance on such forward-looking statements, which speak only as of the date of this presentation. The Company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. All product names, logos, brands and company names are trademarks or registered trademarks of their respective owners. Their use does not imply affiliation or endorsement by these companies. Forward-Looking Statements
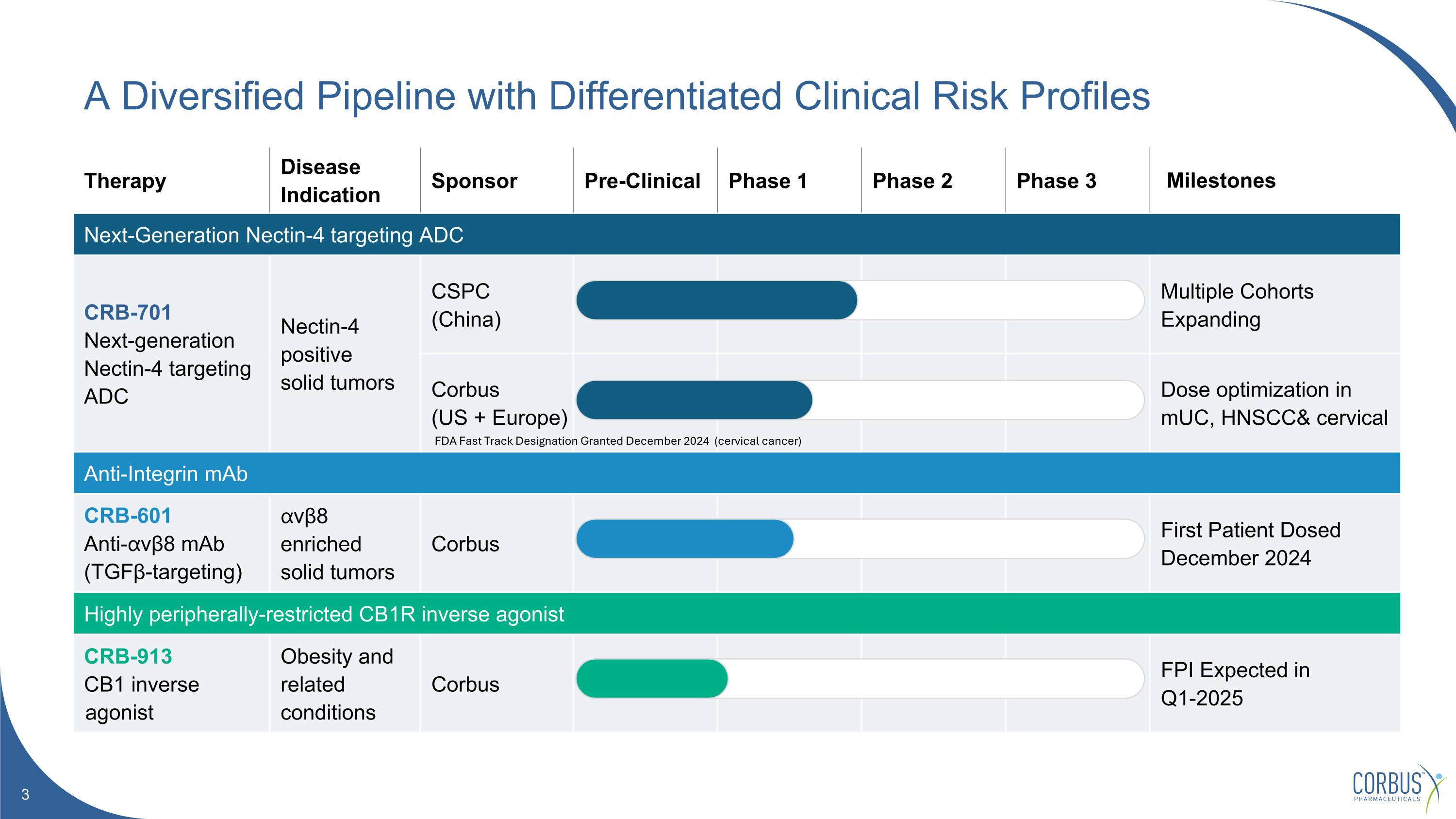
Therapy Disease Indication Sponsor Pre-Clinical Phase 1 Phase 2 Phase 3 Milestones Next-Generation Nectin-4 targeting ADC CRB-701 Next-generation Nectin-4 targeting ADC Nectin-4 positive solid tumors CSPC (China) Multiple Cohorts Expanding Corbus (US + Europe) Dose optimization in mUC, HNSCC& cervical Anti-Integrin mAb CRB-601 Anti-⍺vβ8 mAb (TGFβ-targeting) ⍺vβ8 enriched solid tumors Corbus First Patient Dosed December 2024 Highly peripherally-restricted CB1R inverse agonist CRB-913 CB1 inverse agonist Obesity and related conditions Corbus FPI Expected in Q1-2025 A Diversified Pipeline with Differentiated Clinical Risk Profiles FDA Fast Track Designation Granted December 2024 (cervical cancer)

CRB-701 Next Generation Nectin-4 Targeting ADC
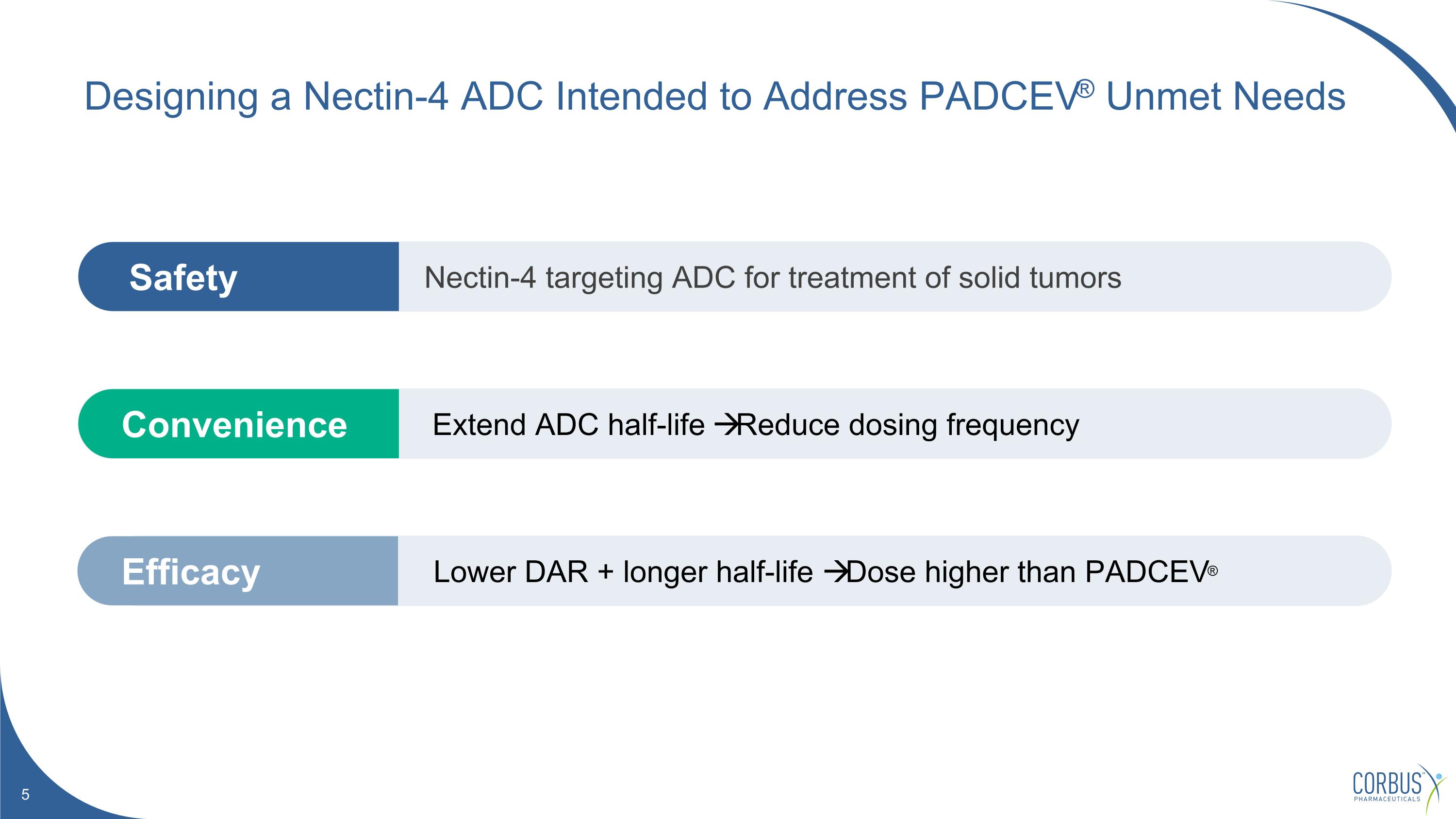
Extend ADC half-life Reduce dosing frequency Convenience Lower DAR + longer half-life Dose higher than PADCEV® Efficacy Nectin-4 targeting ADC for treatment of solid tumors Safety Designing a Nectin-4 ADC Intended to Address PADCEV® Unmet Needs
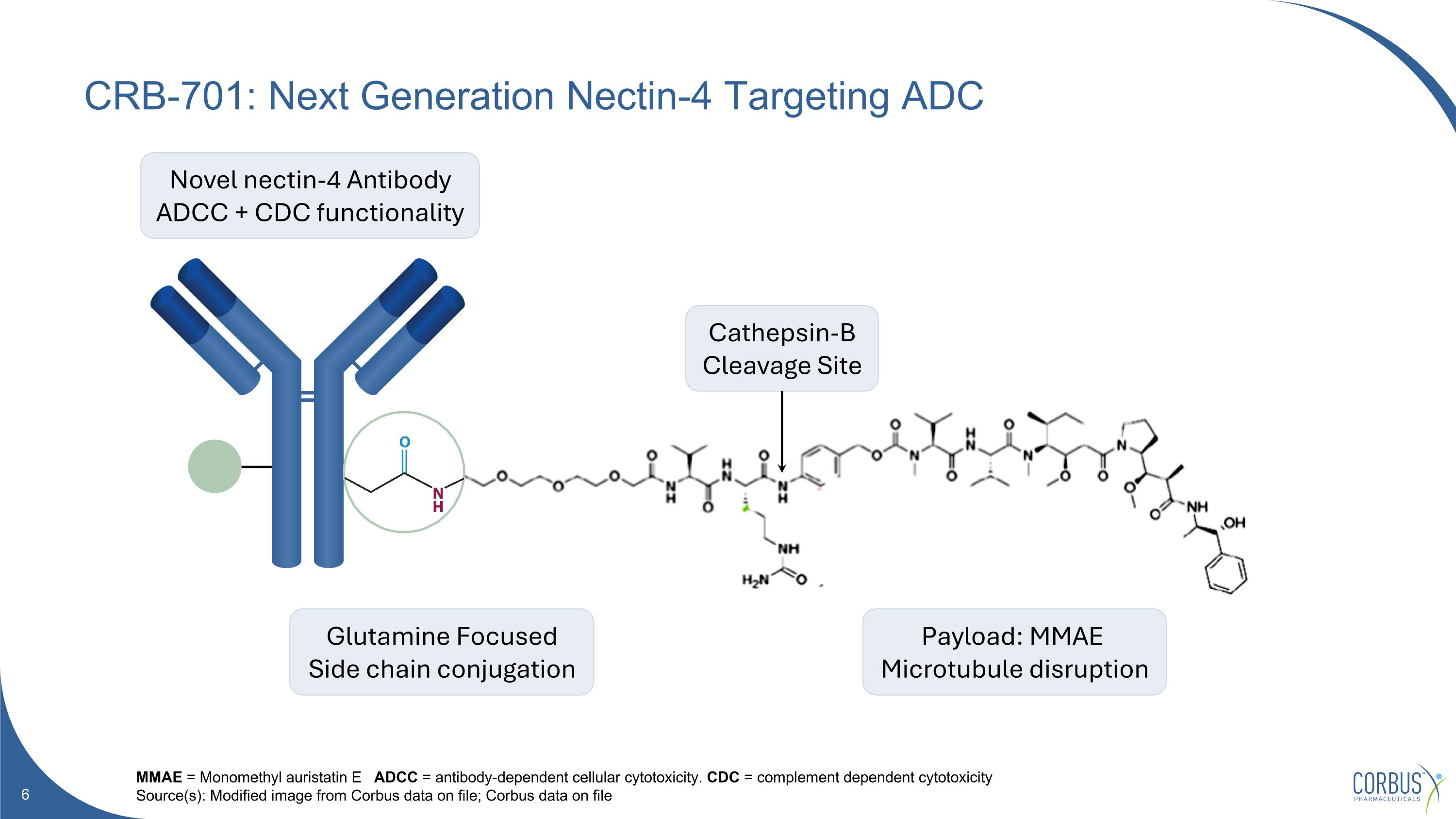
CRB-701: Next Generation Nectin-4 Targeting ADC MMAE = Monomethyl auristatin E ADCC = antibody-dependent cellular cytotoxicity. CDC = complement dependent cytotoxicity Source(s): Modified image from Corbus data on file; Corbus data on file Novel nectin-4 Antibody ADCC + CDC functionality Glutamine Focused Side chain conjugation Payload: MMAE Microtubule disruption Cathepsin-B Cleavage Site
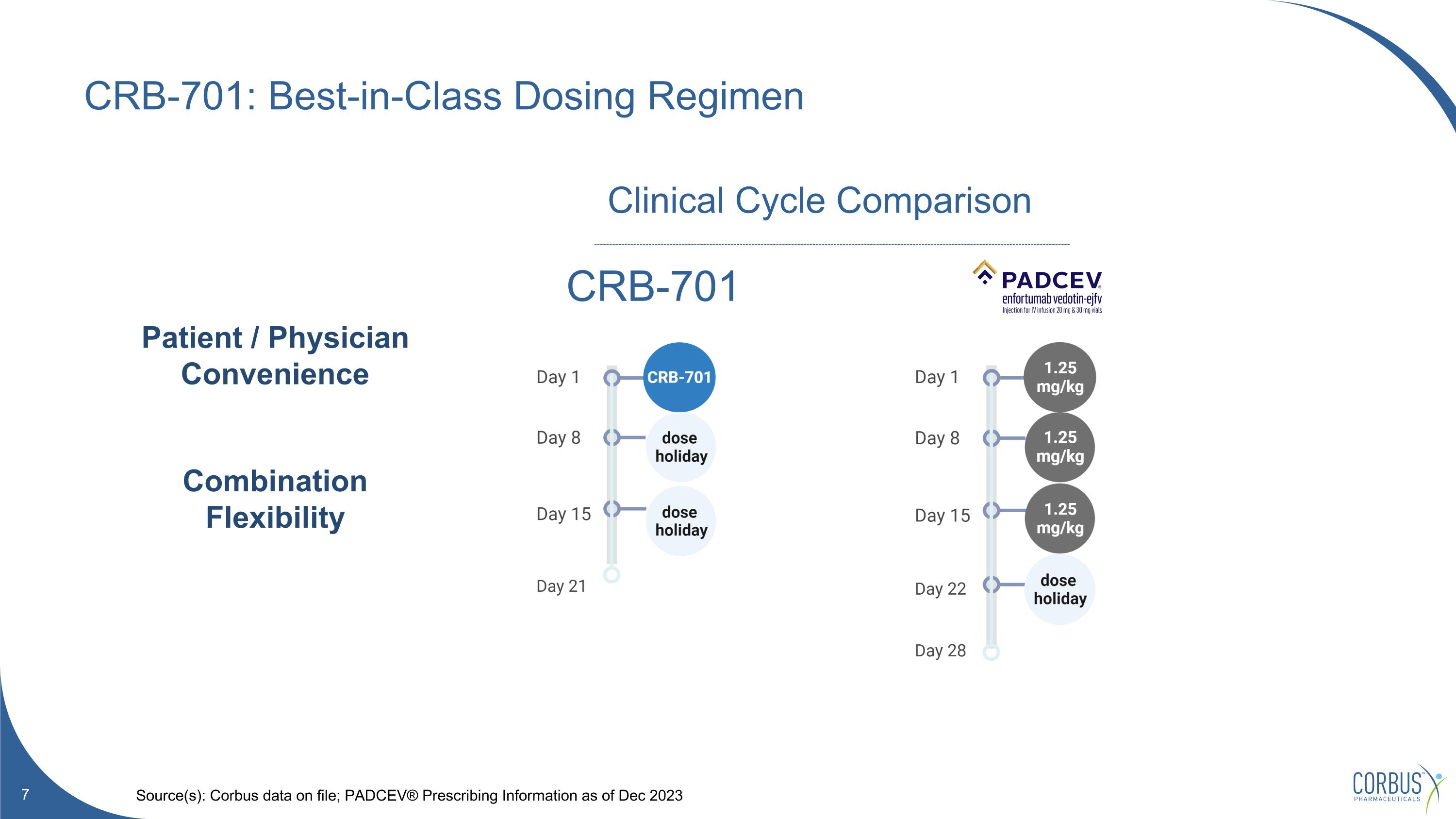
CRB-701: Best-in-Class Dosing Regimen Source(s): Corbus data on file; PADCEV® Prescribing Information as of Dec 2023 CRB-701 Clinical Cycle Comparison Patient / Physician Convenience Combination Flexibility

Phase 1 Dose Escalation Studies: Trial Design KEY ELIGIBILITY Age ≥ 18 years Advanced urothelial carcinoma or Nectin-4 positive Advanced solid tumors ECOG 0-1 Adequate organ function Stable ongoing comorbidities No active CNS metastasis ESCALATION DESIGN Bayesian Optimal Interval (BOIN) design with accelerated titration at DL-1 IV Q3W over a 21-day cycle 0.2 mg/kg 0.6 mg/kg 1.2 mg/kg 1.8 mg/kg 2.7 mg/kg (expanding) 3.6 mg/kg (expanding) 4.5 mg/kg KEY ENDPOINTS Safety/tolerability PK and Efficacy NEXT STEPS Continued expansion at 2 doses ESCALATION DESIGN Bayesian Optimal Interval (BOIN) design with accelerated titration at DL-1 IV Q3W over a 21-day cycle 1.8 mg/kg 2.7 mg/kg (dose optimization) 3.6 mg/kg (dose optimization) 4.5 mg/kg NEXT STEPS Dose optimization (Project Optimus) monotherapy in HNSCC, cervical, and bladder tumors: PD-1 combo cohorts 2025
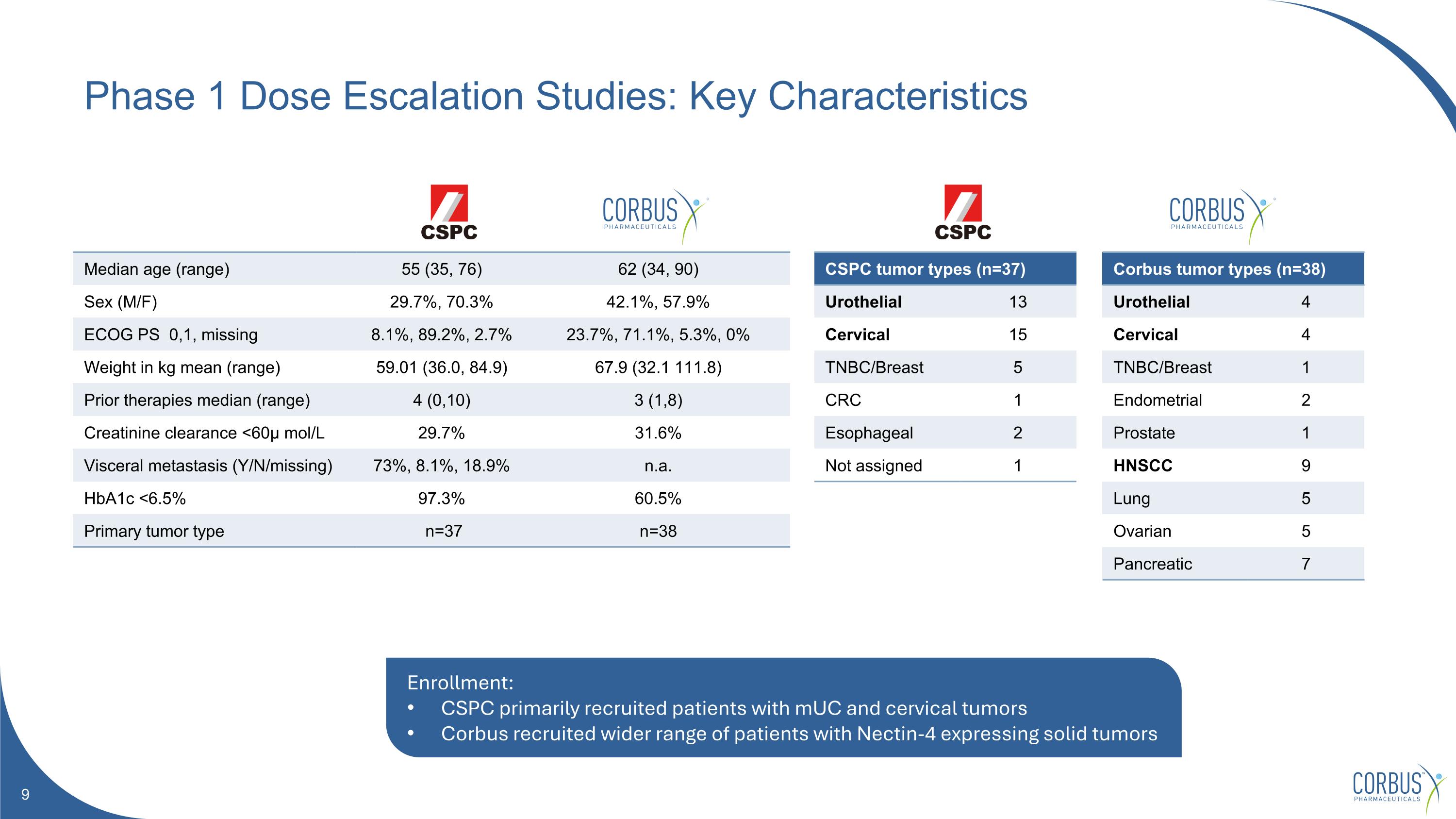
Phase 1 Dose Escalation Studies: Key Characteristics Median age (range) 55 (35, 76) 62 (34, 90) Sex (M/F) 29.7%, 70.3% 42.1%, 57.9% ECOG PS 0,1, missing 8.1%, 89.2%, 2.7% 23.7%, 71.1%, 5.3%, 0% Weight in kg mean (range) 59.01 (36.0, 84.9) 67.9 (32.1 111.8) Prior therapies median (range) 4 (0,10) 3 (1,8) Creatinine clearance <60μ mol/L 29.7% 31.6% Visceral metastasis (Y/N/missing) 73%, 8.1%, 18.9% n.a. HbA1c <6.5% 97.3% 60.5% Primary tumor type n=37 n=38 Corbus tumor types (n=38) Urothelial 4 Cervical 4 TNBC/Breast 1 Endometrial 2 Prostate 1 HNSCC 9 Lung 5 Ovarian 5 Pancreatic 7 CSPC tumor types (n=37) Urothelial 13 Cervical 15 TNBC/Breast 5 CRC 1 Esophageal 2 Not assigned 1 Enrollment: CSPC primarily recruited patients with mUC and cervical tumors Corbus recruited wider range of patients with Nectin-4 expressing solid tumors
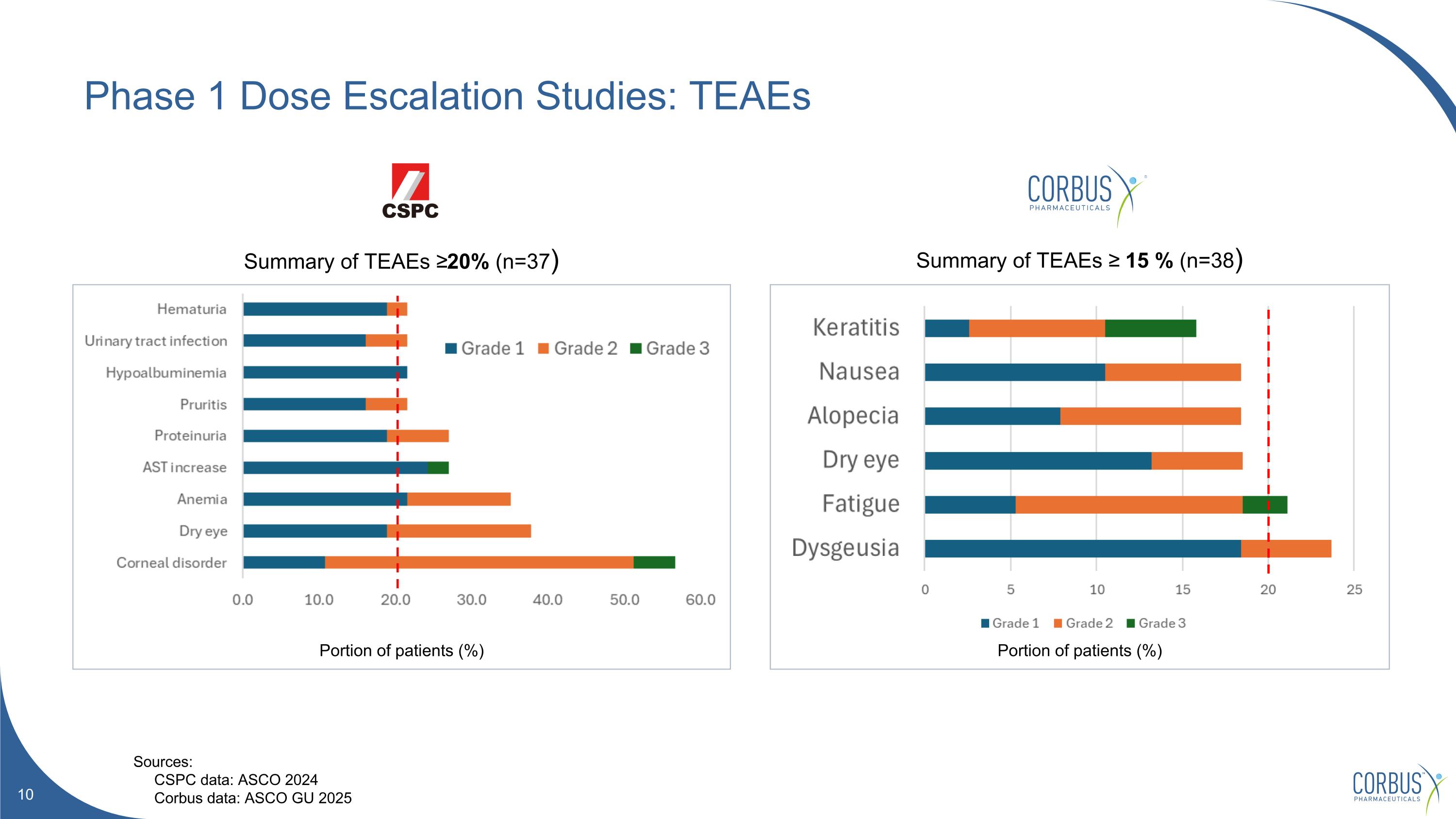
Phase 1 Dose Escalation Studies: TEAEs Summary of TEAEs ≥20% (n=37) Summary of TEAEs ≥ 15 % (n=38) Portion of patients (%) Portion of patients (%) Sources: CSPC data: ASCO 2024 Corbus data: ASCO GU 2025
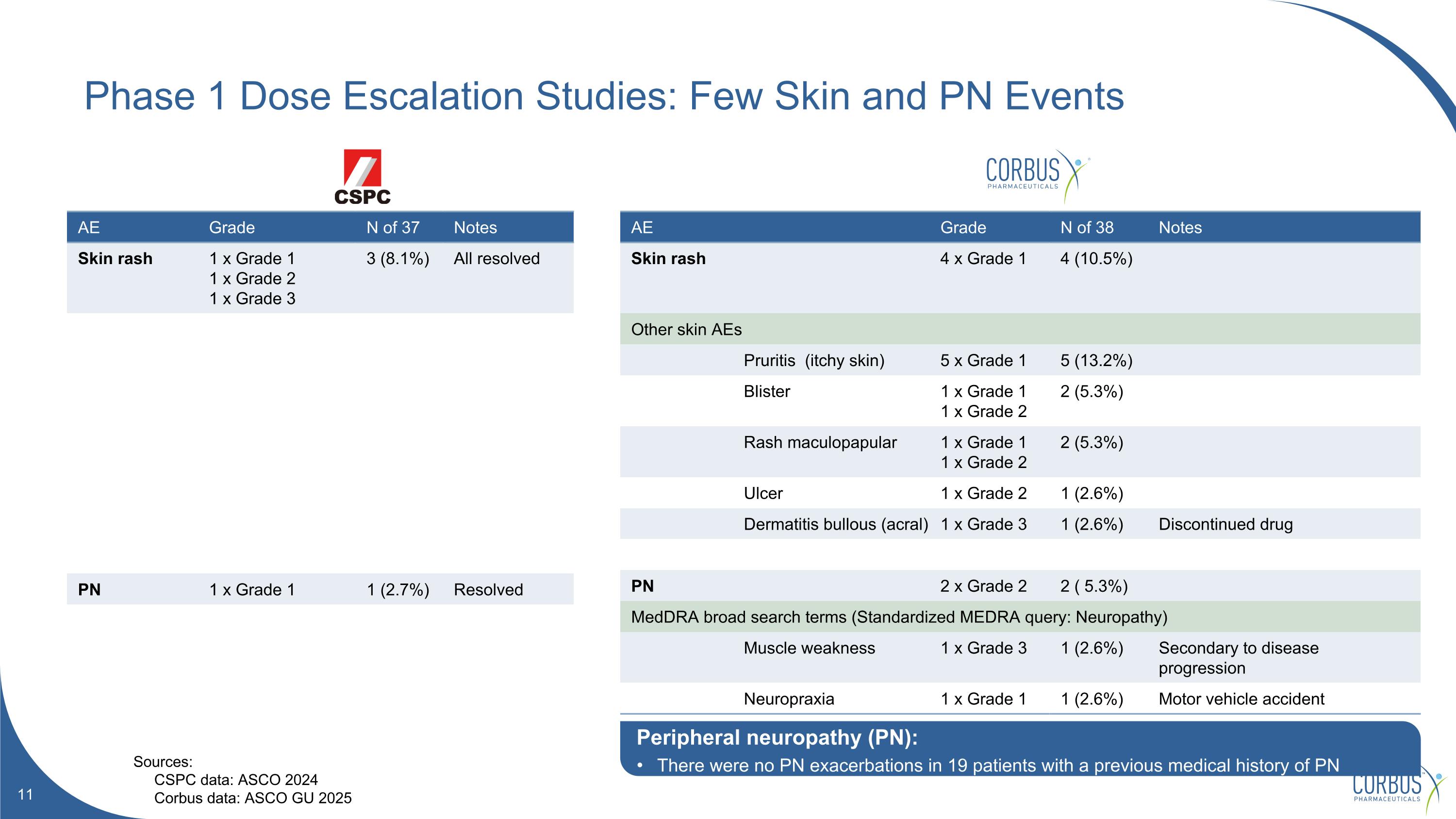
AE Grade N of 37 Notes Skin rash 1 x Grade 1 1 x Grade 2 1 x Grade 3 3 (8.1%) All resolved PN 1 x Grade 1 1 (2.7%) Resolved AE Grade N of 38 Notes Skin rash 4 x Grade 1 4 (10.5%) Other skin AEs Pruritis (itchy skin) 5 x Grade 1 5 (13.2%) Blister 1 x Grade 1 1 x Grade 2 2 (5.3%) Rash maculopapular 1 x Grade 1 1 x Grade 2 2 (5.3%) Ulcer 1 x Grade 2 1 (2.6%) Dermatitis bullous (acral) 1 x Grade 3 1 (2.6%) Discontinued drug PN 2 x Grade 2 2 ( 5.3%) MedDRA broad search terms (Standardized MEDRA query: Neuropathy) Muscle weakness 1 x Grade 3 1 (2.6%) Secondary to disease progression Neuropraxia 1 x Grade 1 1 (2.6%) Motor vehicle accident Phase 1 Dose Escalation Studies: Few Skin and PN Events Sources: CSPC data: ASCO 2024 Corbus data: ASCO GU 2025 Peripheral neuropathy (PN): There were no PN exacerbations in 19 patients with a previous medical history of PN
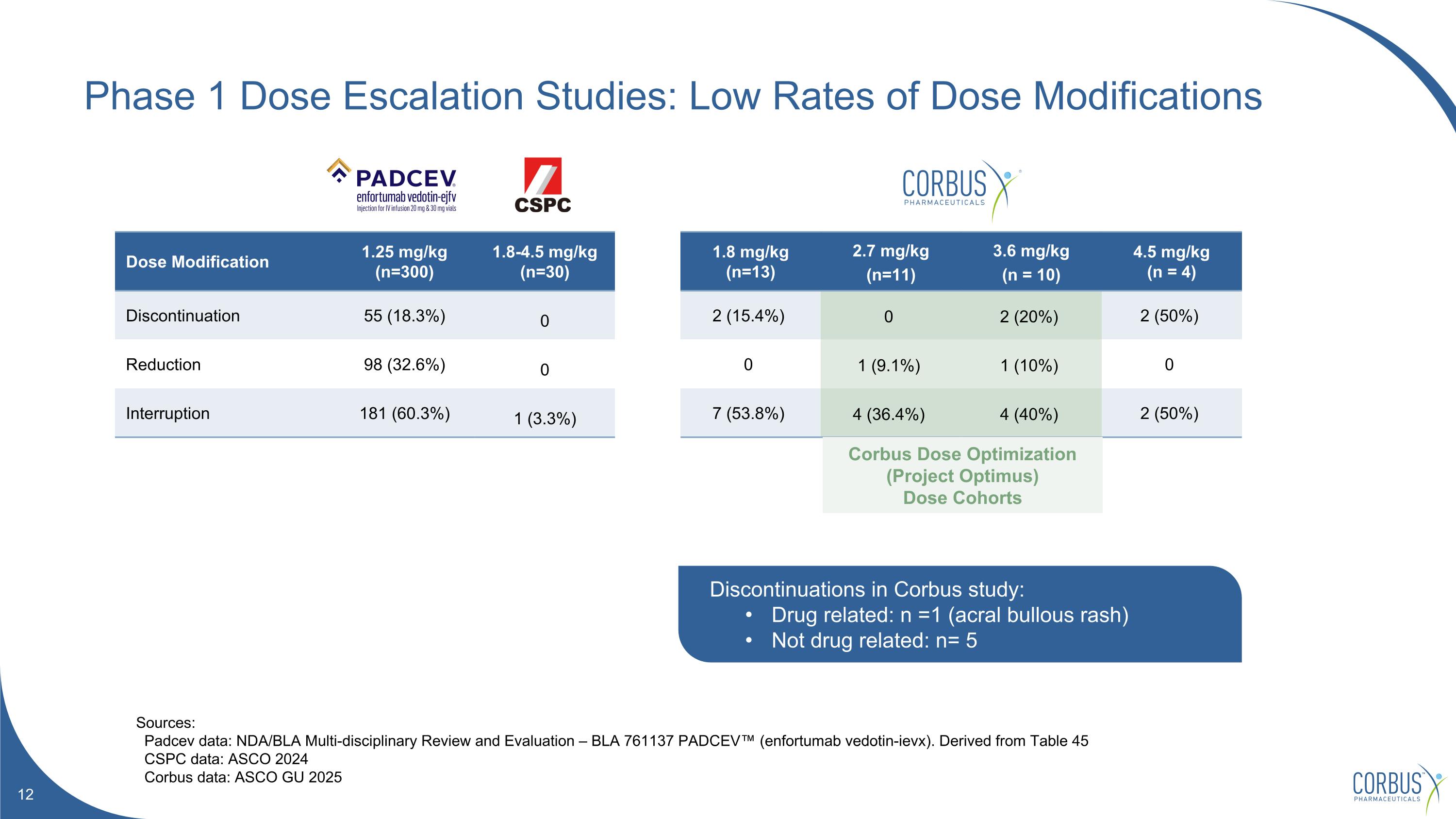
Phase 1 Dose Escalation Studies: Low Rates of Dose Modifications Sources: Padcev data: NDA/BLA Multi-disciplinary Review and Evaluation – BLA 761137 PADCEV™ (enfortumab vedotin-ievx). Derived from Table 45 CSPC data: ASCO 2024 Corbus data: ASCO GU 2025 Dose Modification 1.25 mg/kg (n=300) 1.8-4.5 mg/kg (n=30) 1.8 mg/kg (n=13) 2.7 mg/kg (n=11) 3.6 mg/kg (n = 10) 4.5 mg/kg (n = 4) Discontinuation 55 (18.3%) 0 2 (15.4%) 0 2 (20%) 2 (50%) Reduction 98 (32.6%) 0 0 1 (9.1%) 1 (10%) 0 Interruption 181 (60.3%) 1 (3.3%) 7 (53.8%) 4 (36.4%) 4 (40%) 2 (50%) Discontinuations in Corbus study: Drug related: n =1 (acral bullous rash) Not drug related: n= 5 Corbus Dose Optimization (Project Optimus) Dose Cohorts
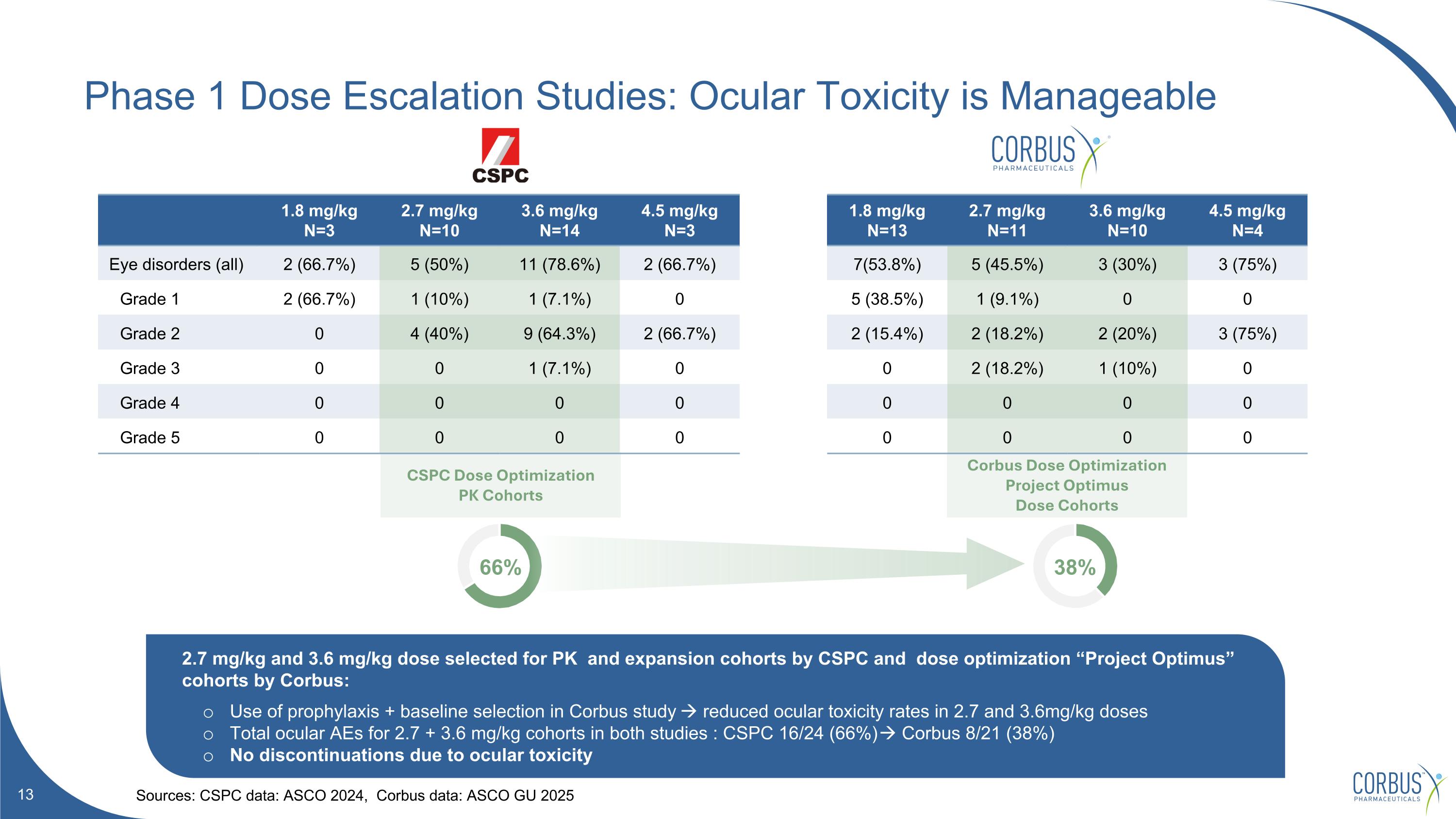
CSPC Dose Optimization PK Cohorts Corbus Dose Optimization Project Optimus Dose Cohorts Phase 1 Dose Escalation Studies: Ocular Toxicity is Manageable Sources: CSPC data: ASCO 2024, Corbus data: ASCO GU 2025 1.8 mg/kg N=3 2.7 mg/kg N=10 3.6 mg/kg N=14 4.5 mg/kg N=3 1.8 mg/kg N=13 2.7 mg/kg N=11 3.6 mg/kg N=10 4.5 mg/kg N=4 Eye disorders (all) 2 (66.7%) 5 (50%) 11 (78.6%) 2 (66.7%) 7(53.8%) 5 (45.5%) 3 (30%) 3 (75%) Grade 1 2 (66.7%) 1 (10%) 1 (7.1%) 0 5 (38.5%) 1 (9.1%) 0 0 Grade 2 0 4 (40%) 9 (64.3%) 2 (66.7%) 2 (15.4%) 2 (18.2%) 2 (20%) 3 (75%) Grade 3 0 0 1 (7.1%) 0 0 2 (18.2%) 1 (10%) 0 Grade 4 0 0 0 0 0 0 0 0 Grade 5 0 0 0 0 0 0 0 0 2.7 mg/kg and 3.6 mg/kg dose selected for PK and expansion cohorts by CSPC and dose optimization “Project Optimus” cohorts by Corbus: Use of prophylaxis + baseline selection in Corbus study reduced ocular toxicity rates in 2.7 and 3.6mg/kg doses Total ocular AEs for 2.7 + 3.6 mg/kg cohorts in both studies : CSPC 16/24 (66%) Corbus 8/21 (38%) No discontinuations due to ocular toxicity 66% 38%
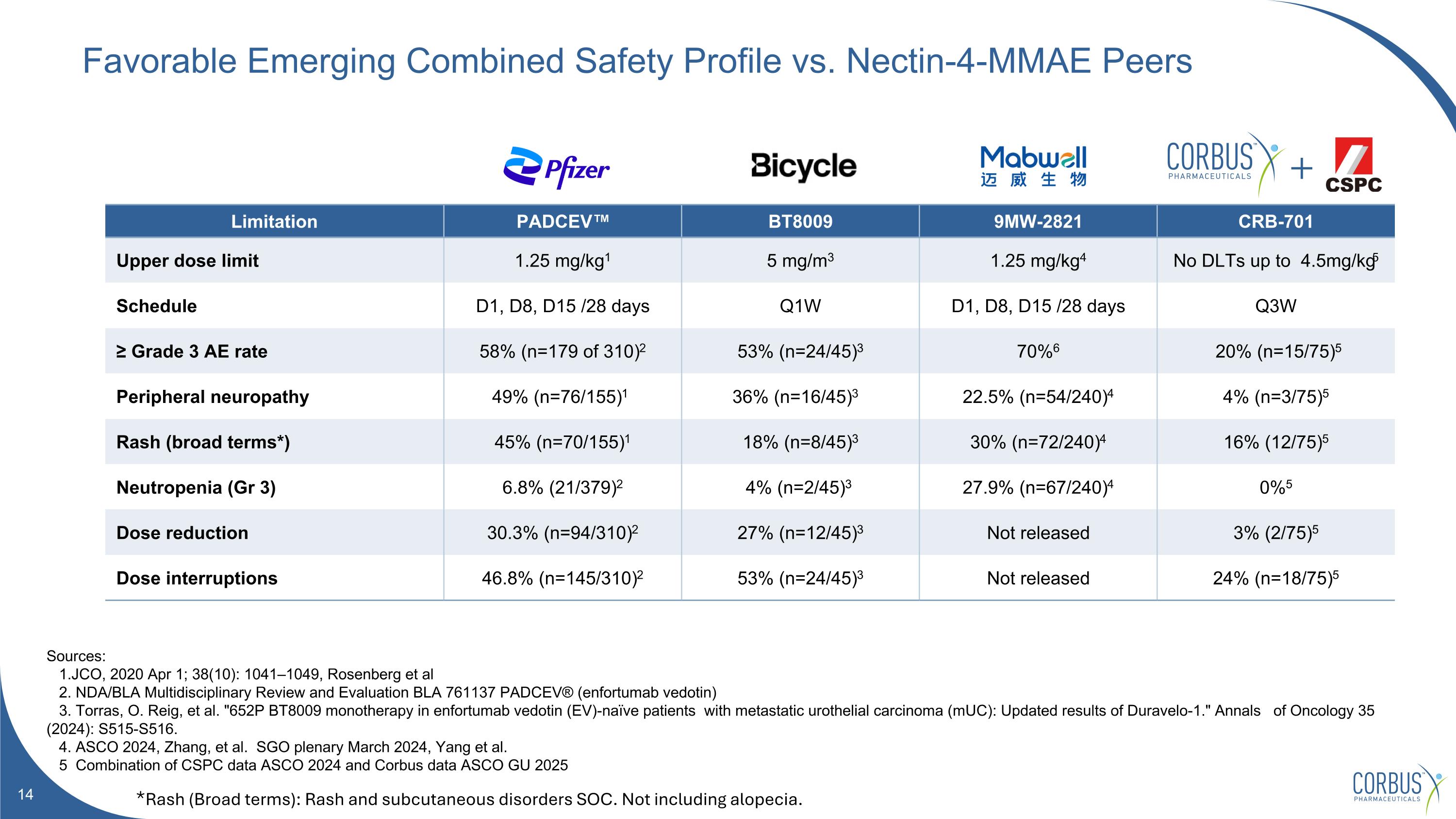
Favorable Emerging Combined Safety Profile vs. Nectin-4-MMAE Peers Sources: 1.JCO, 2020 Apr 1; 38(10): 1041–1049, Rosenberg et al 2. NDA/BLA Multidisciplinary Review and Evaluation BLA 761137 PADCEV® (enfortumab vedotin) 3. Torras, O. Reig, et al. "652P BT8009 monotherapy in enfortumab vedotin (EV)-naïve patients with metastatic urothelial carcinoma (mUC): Updated results of Duravelo-1." Annals of Oncology 35 (2024): S515-S516. 4. ASCO 2024, Zhang, et al. SGO plenary March 2024, Yang et al. 5 Combination of CSPC data ASCO 2024 and Corbus data ASCO GU 2025 Limitation PADCEV™ BT8009 9MW-2821 CRB-701 Upper dose limit 1.25 mg/kg1 5 mg/m3 1.25 mg/kg4 No DLTs up to 4.5mg/kg5 Schedule D1, D8, D15 /28 days Q1W D1, D8, D15 /28 days Q3W ≥ Grade 3 AE rate 58% (n=179 of 310)2 53% (n=24/45)3 70%6 20% (n=15/75)5 Peripheral neuropathy 49% (n=76/155)1 36% (n=16/45)3 22.5% (n=54/240)4 4% (n=3/75)5 Rash (broad terms*) 45% (n=70/155)1 18% (n=8/45)3 30% (n=72/240)4 16% (12/75)5 Neutropenia (Gr 3) 6.8% (21/379)2 4% (n=2/45)3 27.9% (n=67/240)4 0%5 Dose reduction 30.3% (n=94/310)2 27% (n=12/45)3 Not released 3% (2/75)5 Dose interruptions 46.8% (n=145/310)2 53% (n=24/45)3 Not released 24% (n=18/75)5 *Rash (Broad terms): Rash and subcutaneous disorders SOC. Not including alopecia.
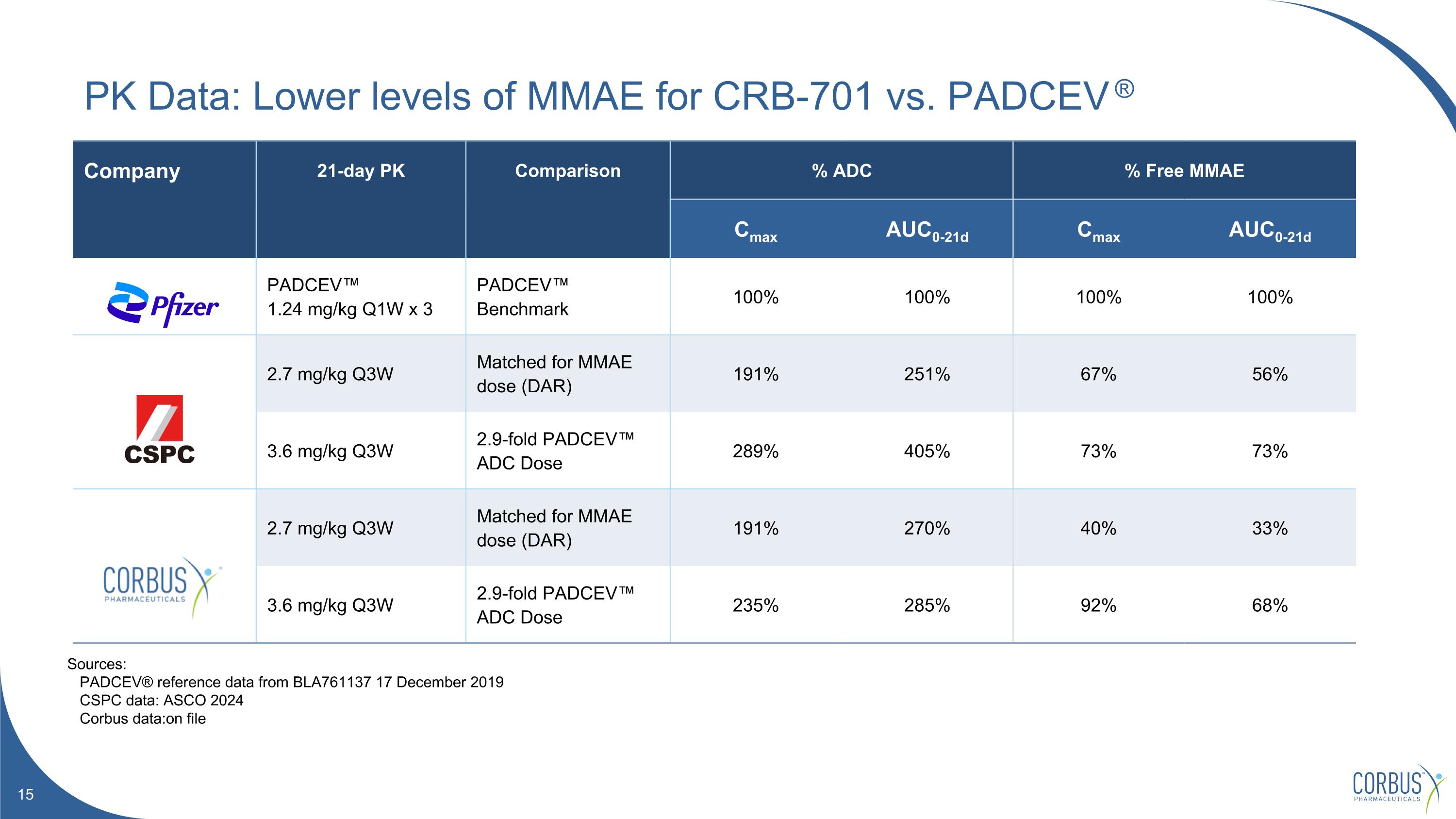
Company 21-day PK Comparison % ADC % Free MMAE Cmax AUC0-21d Cmax AUC0-21d PADCEV™ 1.24 mg/kg Q1W x 3 PADCEV™ Benchmark 100% 100% 100% 100% 2.7 mg/kg Q3W Matched for MMAE dose (DAR) 191% 251% 67% 56% 3.6 mg/kg Q3W 2.9-fold PADCEV™ ADC Dose 289% 405% 73% 73% 2.7 mg/kg Q3W Matched for MMAE dose (DAR) 191% 270% 40% 33% 3.6 mg/kg Q3W 2.9-fold PADCEV™ ADC Dose 235% 285% 92% 68% PK Data: Lower levels of MMAE for CRB-701 vs. PADCEV ® Sources: PADCEV® reference data from BLA761137 17 December 2019 CSPC data: ASCO 2024 Corbus data:on file
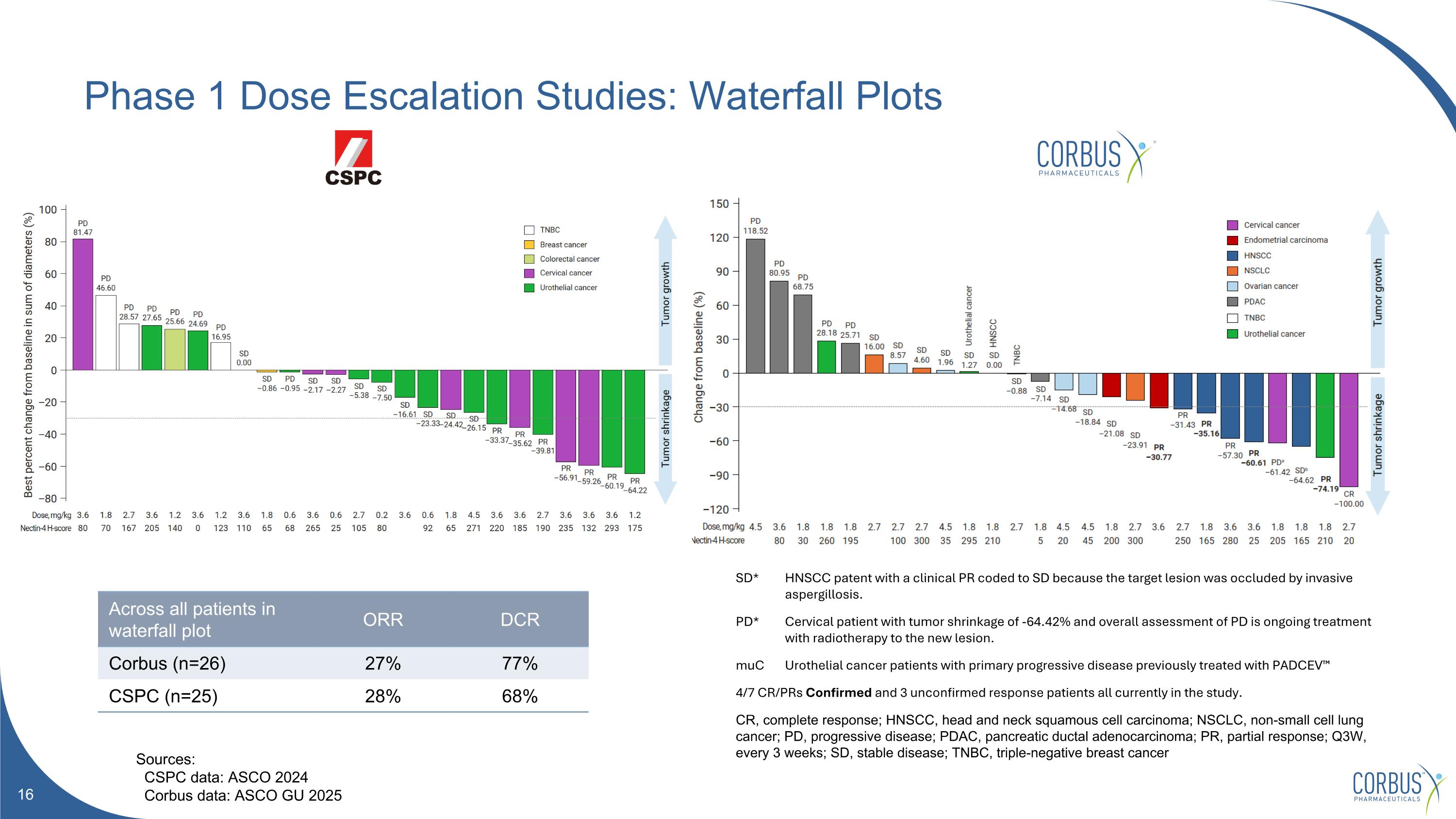
Phase 1 Dose Escalation Studies: Waterfall Plots *confirmed response Across all patients in waterfall plot ORR DCR Corbus (n=26) 27% 77% CSPC (n=25) 28% 68% SD* HNSCC patent with a clinical PR coded to SD because the target lesion was occluded by invasive aspergillosis. PD* Cervical patient with tumor shrinkage of -64.42% and overall assessment of PD is ongoing treatment with radiotherapy to the new lesion. muC Urothelial cancer patients with primary progressive disease previously treated with PADCEV™ 4/7 CR/PRs Confirmed and 3 unconfirmed response patients all currently in the study. CR, complete response; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small cell lung cancer; PD, progressive disease; PDAC, pancreatic ductal adenocarcinoma; PR, partial response; Q3W, every 3 weeks; SD, stable disease; TNBC, triple-negative breast cancer Sources: CSPC data: ASCO 2024 Corbus data: ASCO GU 2025
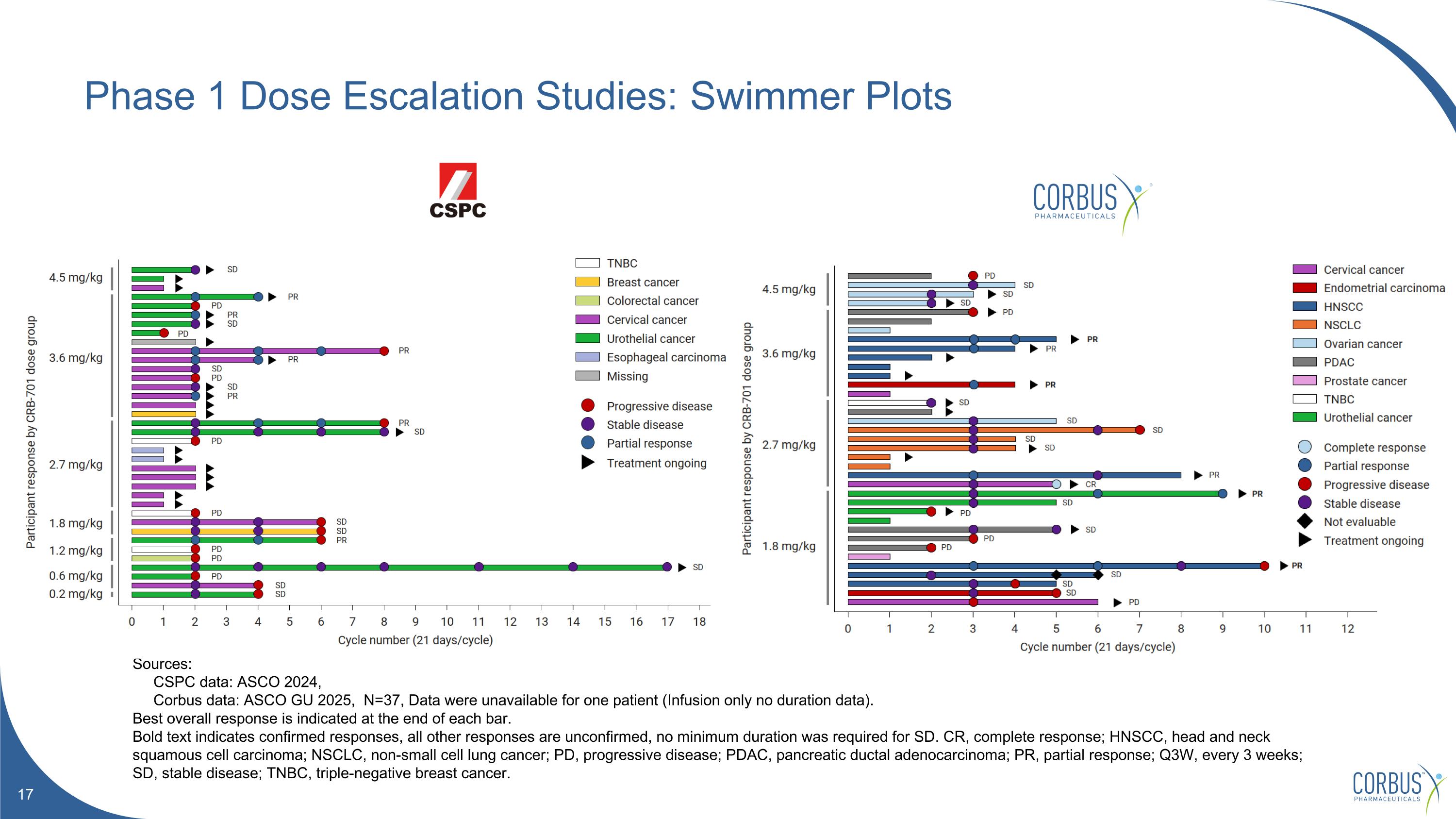
Phase 1 Dose Escalation Studies: Swimmer Plots Sources: CSPC data: ASCO 2024, Corbus data: ASCO GU 2025, N=37, Data were unavailable for one patient (Infusion only no duration data). Best overall response is indicated at the end of each bar. Bold text indicates confirmed responses, all other responses are unconfirmed, no minimum duration was required for SD. CR, complete response; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small cell lung cancer; PD, progressive disease; PDAC, pancreatic ductal adenocarcinoma; PR, partial response; Q3W, every 3 weeks; SD, stable disease; TNBC, triple-negative breast cancer.
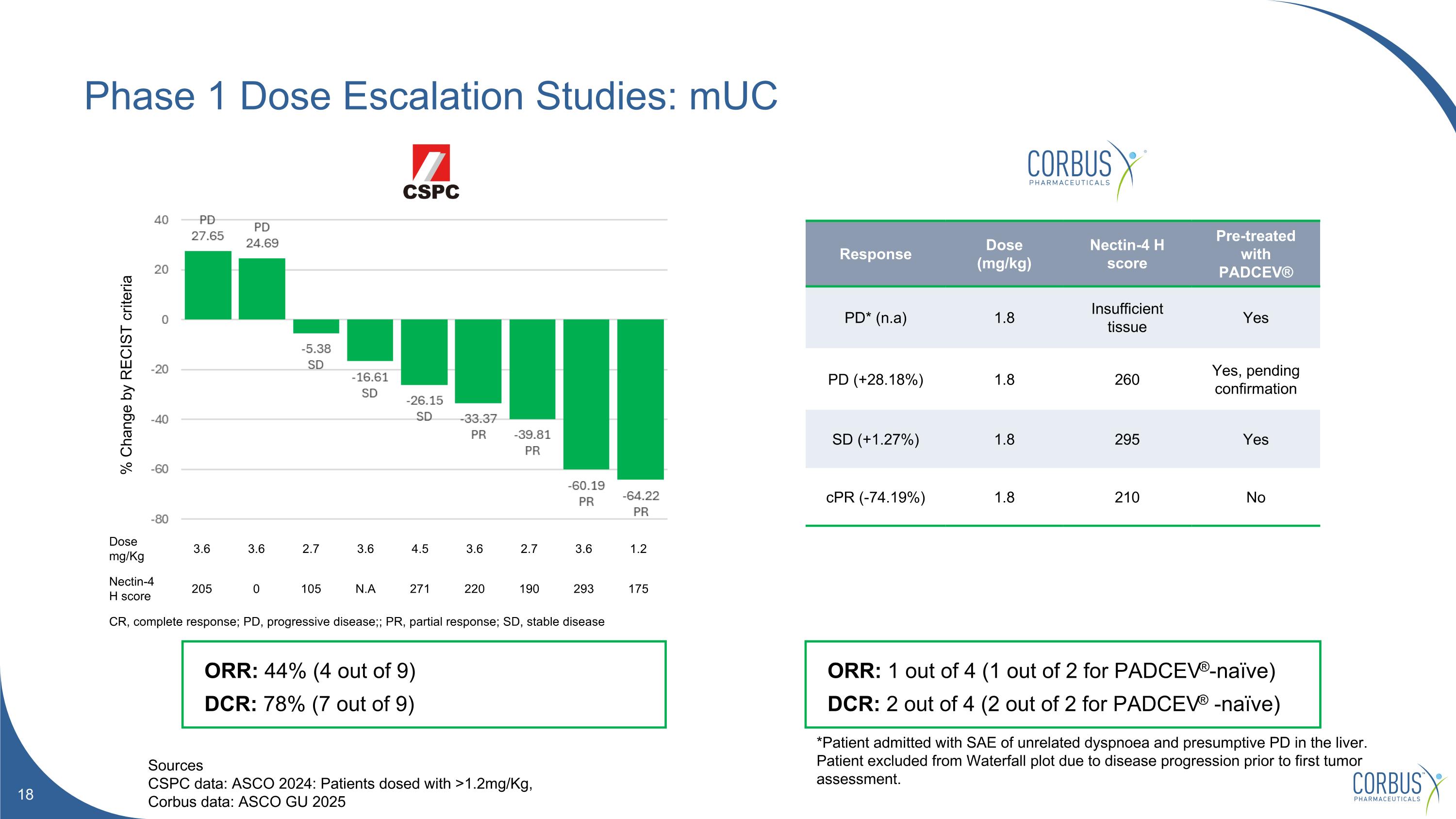
Phase 1 Dose Escalation Studies: mUC Sources CSPC data: ASCO 2024: Patients dosed with >1.2mg/Kg, Corbus data: ASCO GU 2025 *Patient admitted with SAE of unrelated dyspnoea and presumptive PD in the liver. Patient excluded from Waterfall plot due to disease progression prior to first tumor assessment. % Change by RECIST criteria ORR: 44% (4 out of 9) DCR: 78% (7 out of 9) Response Dose (mg/kg) Nectin-4 H score Pre-treated with PADCEV® PD* (n.a) 1.8 Insufficient tissue Yes PD (+28.18%) 1.8 260 Yes, pending confirmation SD (+1.27%) 1.8 295 Yes cPR (-74.19%) 1.8 210 No ORR: 1 out of 4 (1 out of 2 for PADCEV®-naïve) DCR: 2 out of 4 (2 out of 2 for PADCEV® -naïve) Dose mg/Kg 3.6 3.6 2.7 3.6 4.5 3.6 2.7 3.6 1.2 Nectin-4 H score 205 0 105 N.A 271 220 190 293 175 CR, complete response; PD, progressive disease;; PR, partial response; SD, stable disease
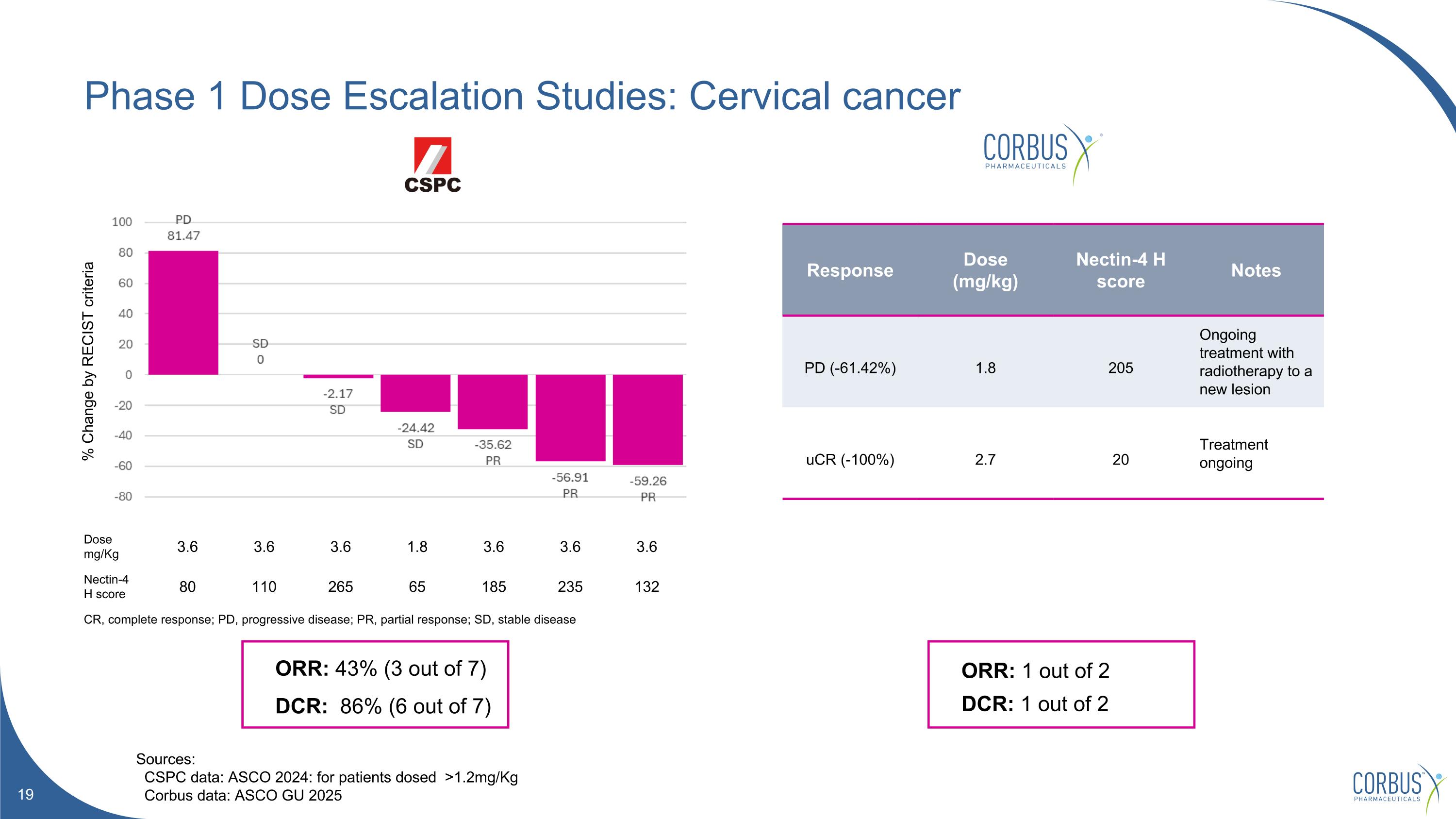
Phase 1 Dose Escalation Studies: Cervical cancer Sources: CSPC data: ASCO 2024: for patients dosed >1.2mg/Kg Corbus data: ASCO GU 2025 ORR: 43% (3 out of 7) DCR: 86% (6 out of 7) ORR: 1 out of 2 DCR: 1 out of 2 Response Dose (mg/kg) Nectin-4 H score Notes PD (-61.42%) 1.8 205 Ongoing treatment with radiotherapy to a new lesion uCR (-100%) 2.7 20 Treatment ongoing % Change by RECIST criteria Dose mg/Kg 3.6 3.6 3.6 1.8 3.6 3.6 3.6 Nectin-4 H score 80 110 265 65 185 235 132 CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease
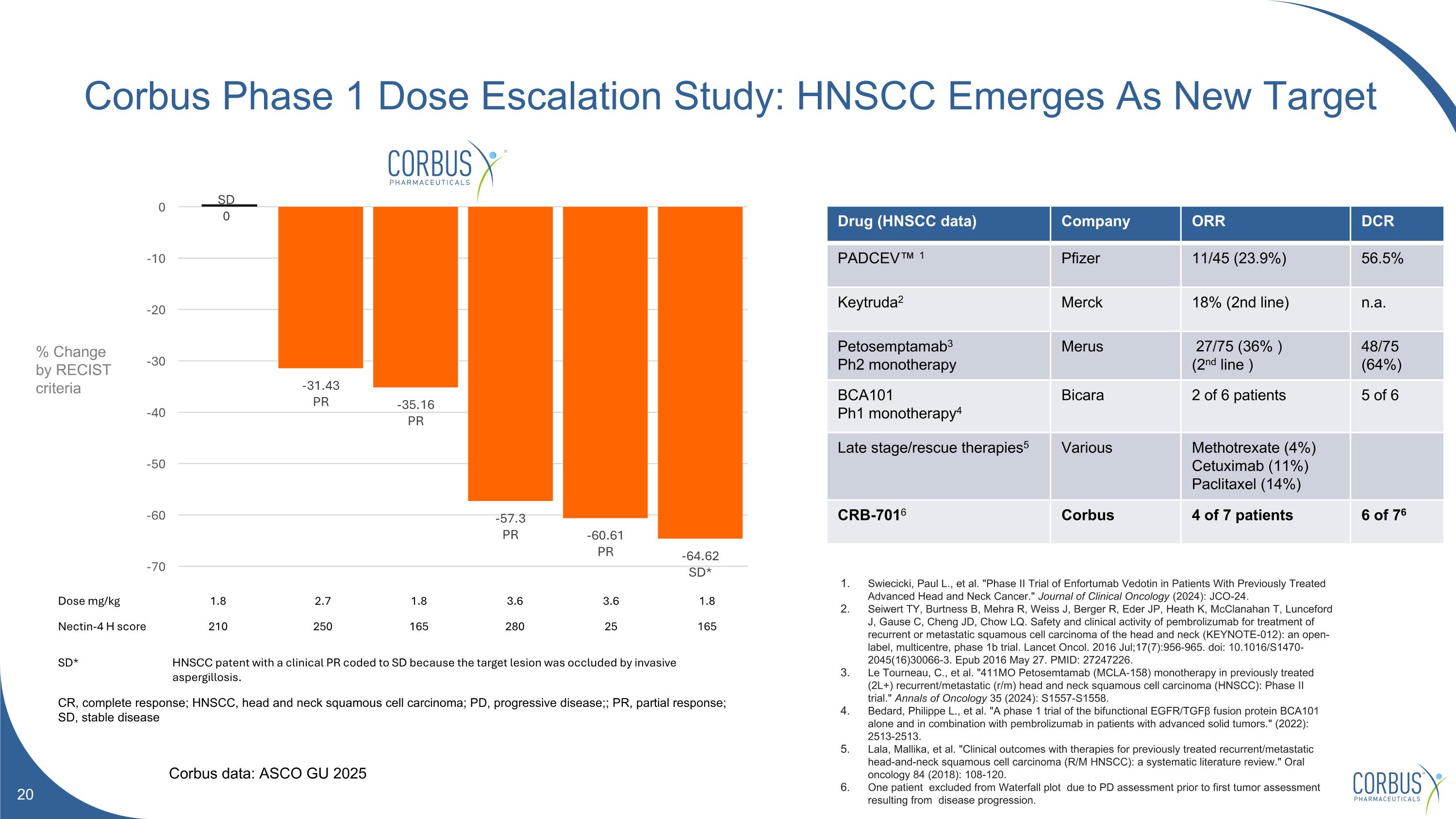
Corbus Phase 1 Dose Escalation Study: HNSCC Emerges As New Target Dose mg/kg 1.8 2.7 1.8 3.6 3.6 1.8 Nectin-4 H score 210 250 165 280 25 165 SD* HNSCC patent with a clinical PR coded to SD because the target lesion was occluded by invasive aspergillosis. CR, complete response; HNSCC, head and neck squamous cell carcinoma; PD, progressive disease;; PR, partial response; SD, stable disease Drug (HNSCC data) Company ORR DCR PADCEV™ 1 Pfizer 11/45 (23.9%) 56.5% Keytruda2 Merck 18% (2nd line) n.a. Petosemptamab3 Ph2 monotherapy Merus 27/75 (36% ) (2nd line ) 48/75 (64%) BCA101 Ph1 monotherapy4 Bicara 2 of 6 patients 5 of 6 Late stage/rescue therapies5 Various Methotrexate (4%) Cetuximab (11%) Paclitaxel (14%) CRB-7016 Corbus 4 of 7 patients 6 of 76 Swiecicki, Paul L., et al. "Phase II Trial of Enfortumab Vedotin in Patients With Previously Treated Advanced Head and Neck Cancer." Journal of Clinical Oncology (2024): JCO-24. Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, Cheng JD, Chow LQ. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016 Jul;17(7):956-965. doi: 10.1016/S1470-2045(16)30066-3. Epub 2016 May 27. PMID: 27247226. Le Tourneau, C., et al. "411MO Petosemtamab (MCLA-158) monotherapy in previously treated (2L+) recurrent/metastatic (r/m) head and neck squamous cell carcinoma (HNSCC): Phase II trial." Annals of Oncology 35 (2024): S1557-S1558. Bedard, Philippe L., et al. "A phase 1 trial of the bifunctional EGFR/TGFβ fusion protein BCA101 alone and in combination with pembrolizumab in patients with advanced solid tumors." (2022): 2513-2513. Lala, Mallika, et al. "Clinical outcomes with therapies for previously treated recurrent/metastatic head-and-neck squamous cell carcinoma (R/M HNSCC): a systematic literature review." Oral oncology 84 (2018): 108-120. One patient excluded from Waterfall plot due to PD assessment prior to first tumor assessment resulting from disease progression. Corbus data: ASCO GU 2025 % Change by RECIST criteria
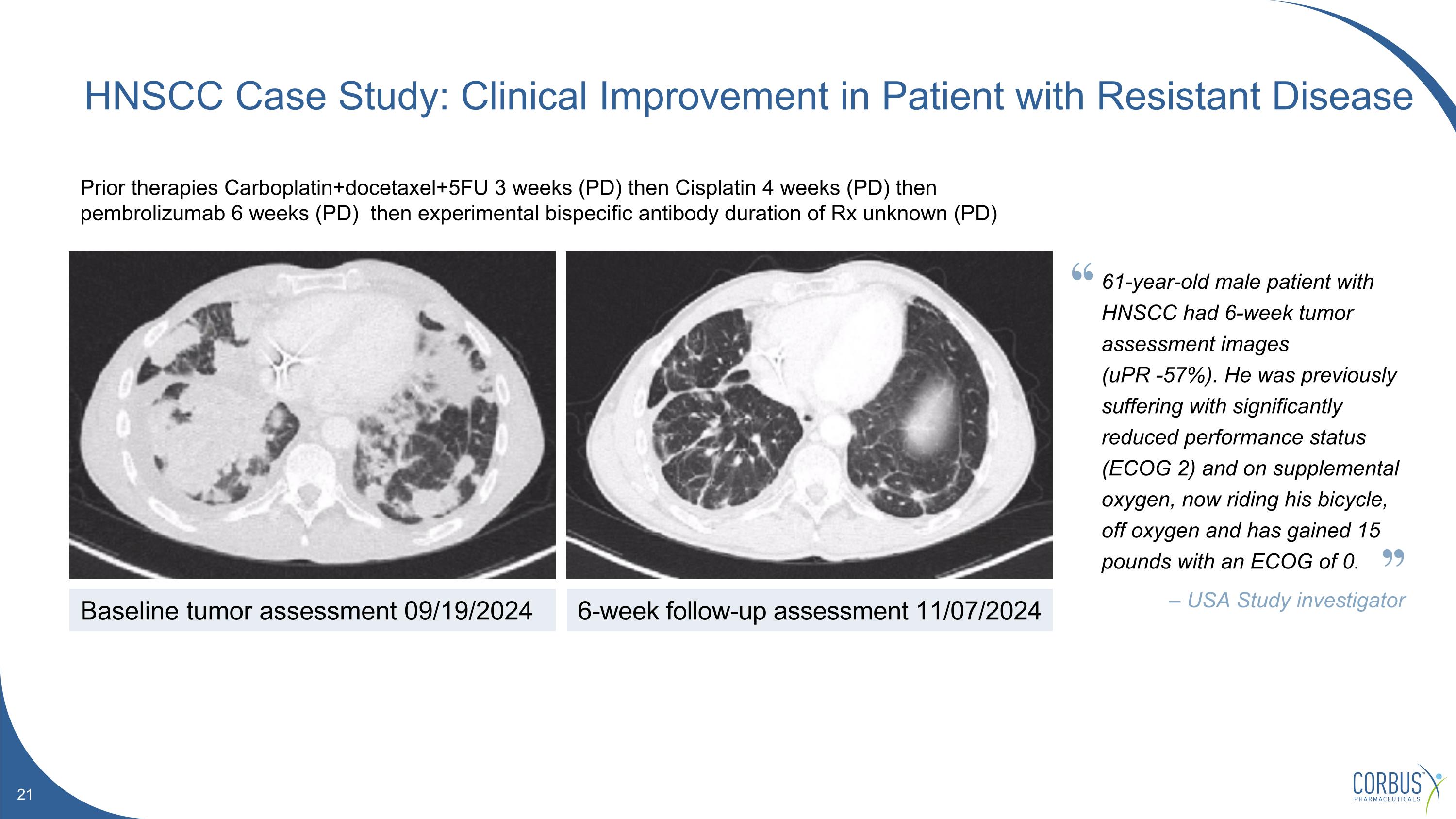
Prior therapies Carboplatin+docetaxel+5FU 3 weeks (PD) then Cisplatin 4 weeks (PD) then pembrolizumab 6 weeks (PD) then experimental bispecific antibody duration of Rx unknown (PD) HNSCC Case Study: Clinical Improvement in Patient with Resistant Disease Baseline tumor assessment 09/19/2024 6-week follow-up assessment 11/07/2024 61-year-old male patient with HNSCC had 6-week tumor assessment images (uPR -57%). He was previously suffering with significantly reduced performance status (ECOG 2) and on supplemental oxygen, now riding his bicycle, off oxygen and has gained 15 pounds with an ECOG of 0. – USA Study investigator
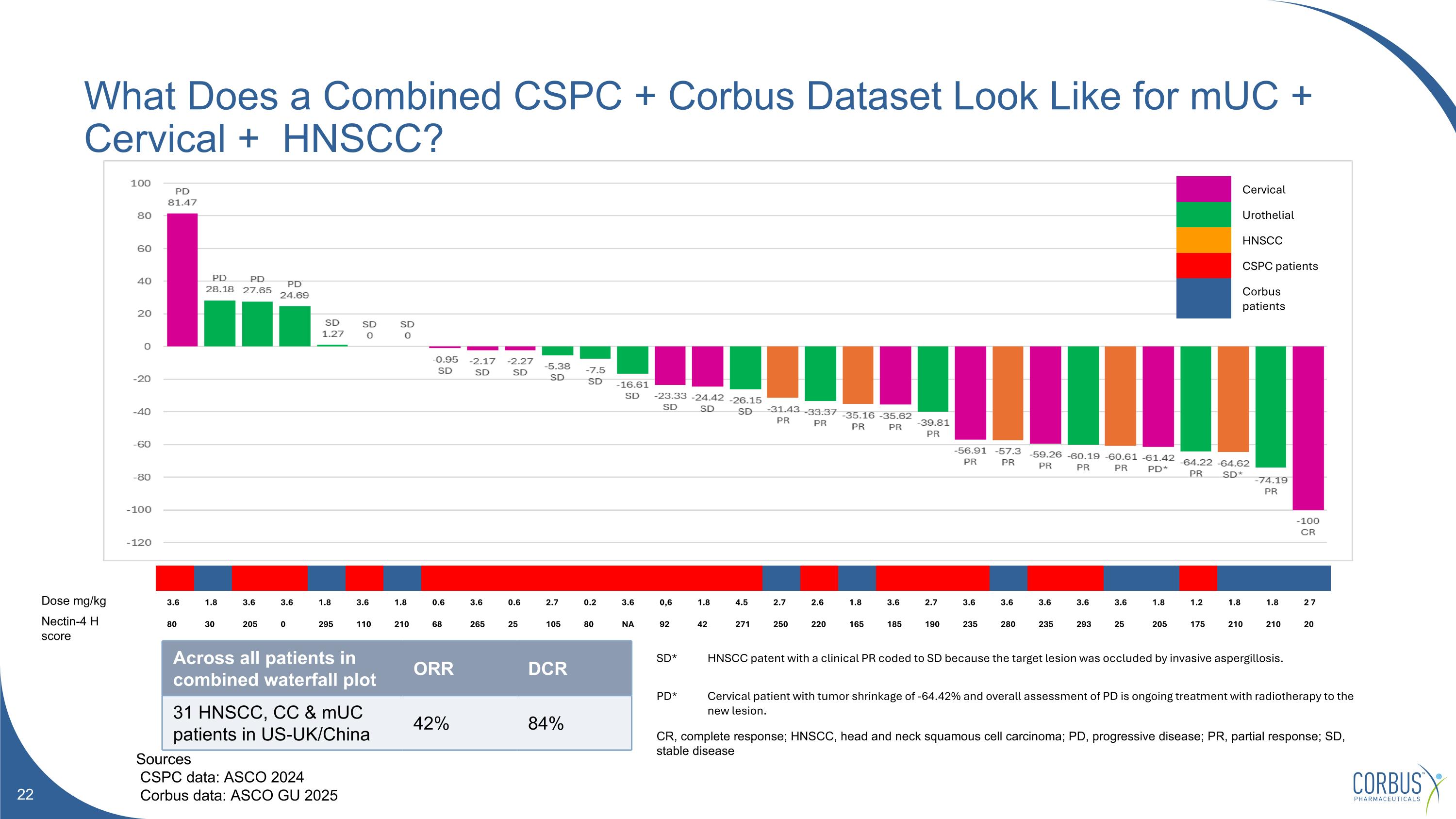
What Does a Combined CSPC + Corbus Dataset Look Like for mUC + Cervical + HNSCC? Sources CSPC data: ASCO 2024 Corbus data: ASCO GU 2025 3.6 1.8 3.6 3.6 1.8 3.6 1.8 0.6 3.6 0.6 2.7 0.2 3.6 0,6 1.8 4.5 2.7 2.6 1.8 3.6 2.7 3.6 3.6 3.6 3.6 3.6 1.8 1.2 1.8 1.8 2 7 80 30 205 0 295 110 210 68 265 25 105 80 NA 92 42 271 250 220 165 185 190 235 280 235 293 25 205 175 210 210 20 Cervical Urothelial HNSCC CSPC patients Corbus patients Across all patients in combined waterfall plot ORR DCR 31 HNSCC, CC & mUC patients in US-UK/China 42% 84% SD* HNSCC patent with a clinical PR coded to SD because the target lesion was occluded by invasive aspergillosis. PD* Cervical patient with tumor shrinkage of -64.42% and overall assessment of PD is ongoing treatment with radiotherapy to the new lesion. CR, complete response; HNSCC, head and neck squamous cell carcinoma; PD, progressive disease; PR, partial response; SD, stable disease Dose mg/kg Nectin-4 H score
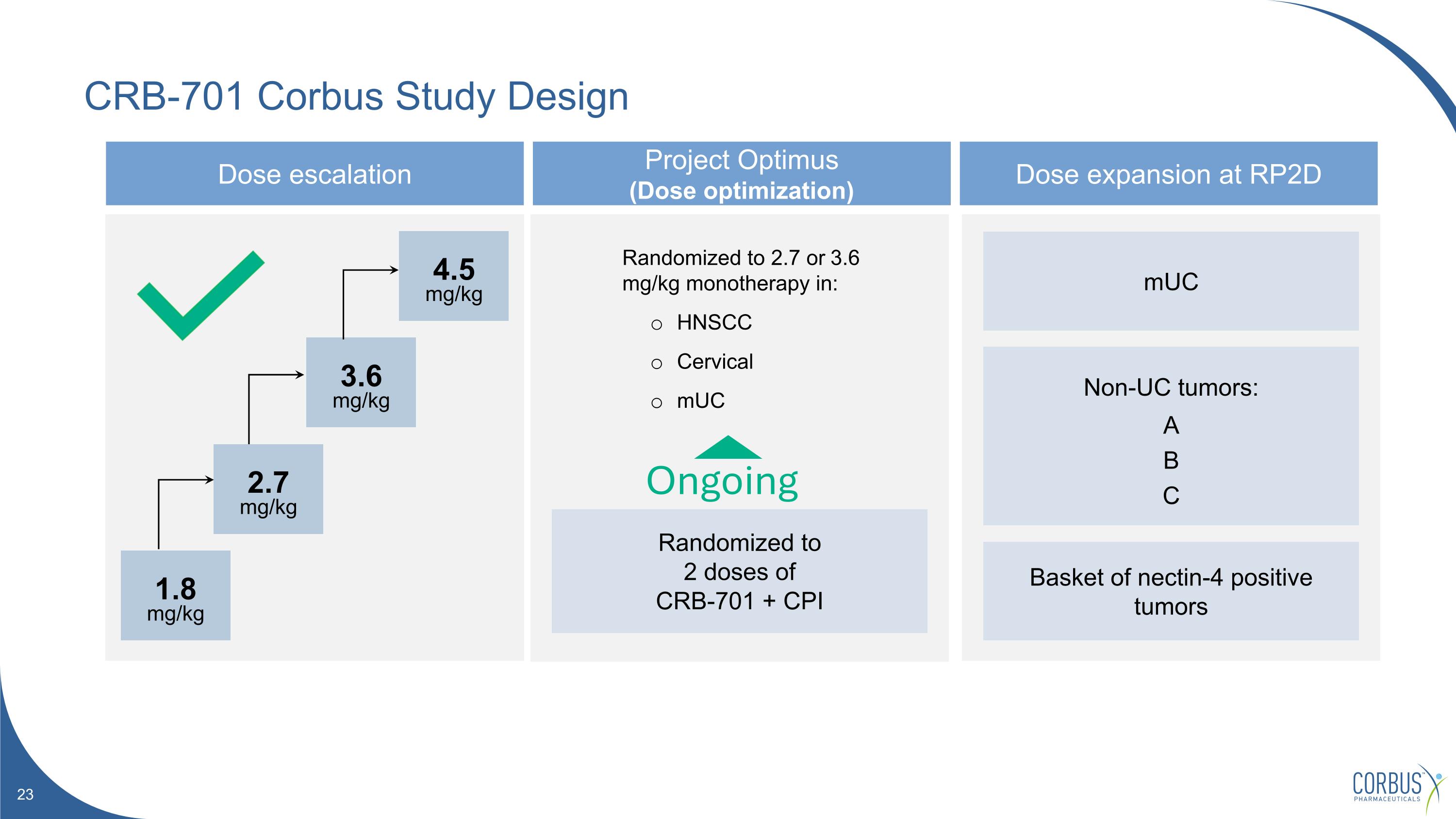
CRB-701 Corbus Study Design Dose escalation Project Optimus (Dose optimization) Dose expansion at RP2D Randomized to 2.7 or 3.6 mg/kg monotherapy in: HNSCC Cervical mUC mUC Non-UC tumors: A B C Basket of nectin-4 positive tumors Ongoing 1.8 mg/kg 2.7 mg/kg 3.6 mg/kg 4.5 mg/kg Randomized to 2 doses of CRB-701 + CPI
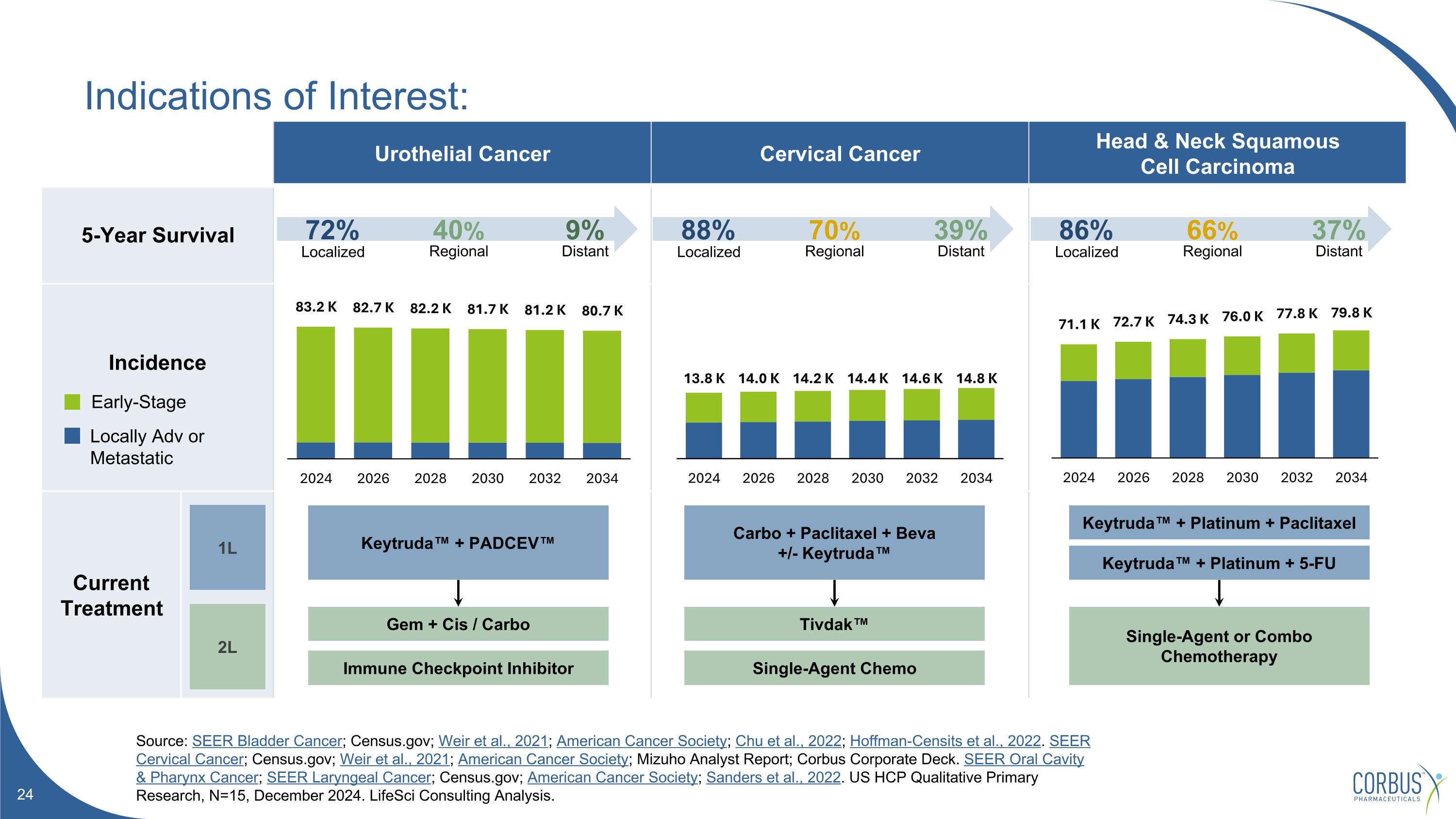
Indications of Interest: Source: SEER Bladder Cancer; Census.gov; Weir et al., 2021; American Cancer Society; Chu et al., 2022; Hoffman-Censits et al., 2022. SEER Cervical Cancer; Census.gov; Weir et al., 2021; American Cancer Society; Mizuho Analyst Report; Corbus Corporate Deck. SEER Oral Cavity & Pharynx Cancer; SEER Laryngeal Cancer; Census.gov; American Cancer Society; Sanders et al., 2022. US HCP Qualitative Primary Research, N=15, December 2024. LifeSci Consulting Analysis. Urothelial Cancer Cervical Cancer Head & Neck Squamous Cell Carcinoma 5-Year Survival Incidence Current Treatment Localized Regional Distant 72% 40% 9% Localized Regional Distant 88% 70% 39% Localized Regional Distant 86% 66% 37% Early-Stage Locally Adv or Metastatic 1L 2L Gem + Cis / Carbo Keytruda™ + PADCEV™ Immune Checkpoint Inhibitor Carbo + Paclitaxel + Beva +/- Keytruda™ Tivdak™ Single-Agent Chemo Keytruda™ + Platinum + Paclitaxel Single-Agent or Combo Chemotherapy Keytruda™ + Platinum + 5-FU
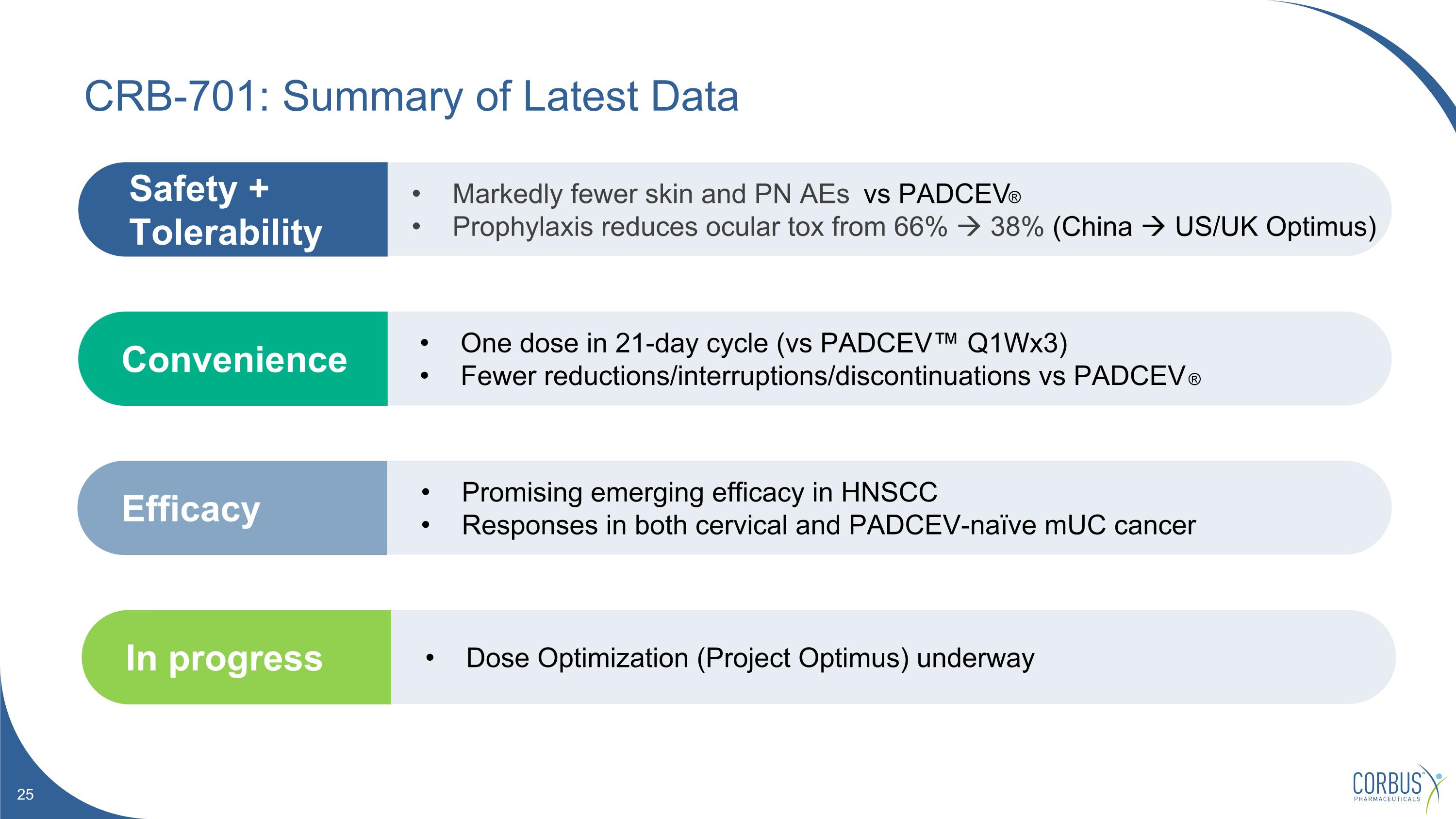
One dose in 21-day cycle (vs PADCEV™ Q1Wx3) Fewer reductions/interruptions/discontinuations vs PADCEV ® Convenience Promising emerging efficacy in HNSCC Responses in both cervical and PADCEV-naïve mUC cancer Efficacy Markedly fewer skin and PN AEs vs PADCEV® Prophylaxis reduces ocular tox from 66% 38% (China US/UK Optimus) Safety + Tolerability CRB-701: Summary of Latest Data Dose Optimization (Project Optimus) underway In progress

CRB-913 Oral cannabinoid Type-1 inverse agonist for superior incretin therapy in obesity
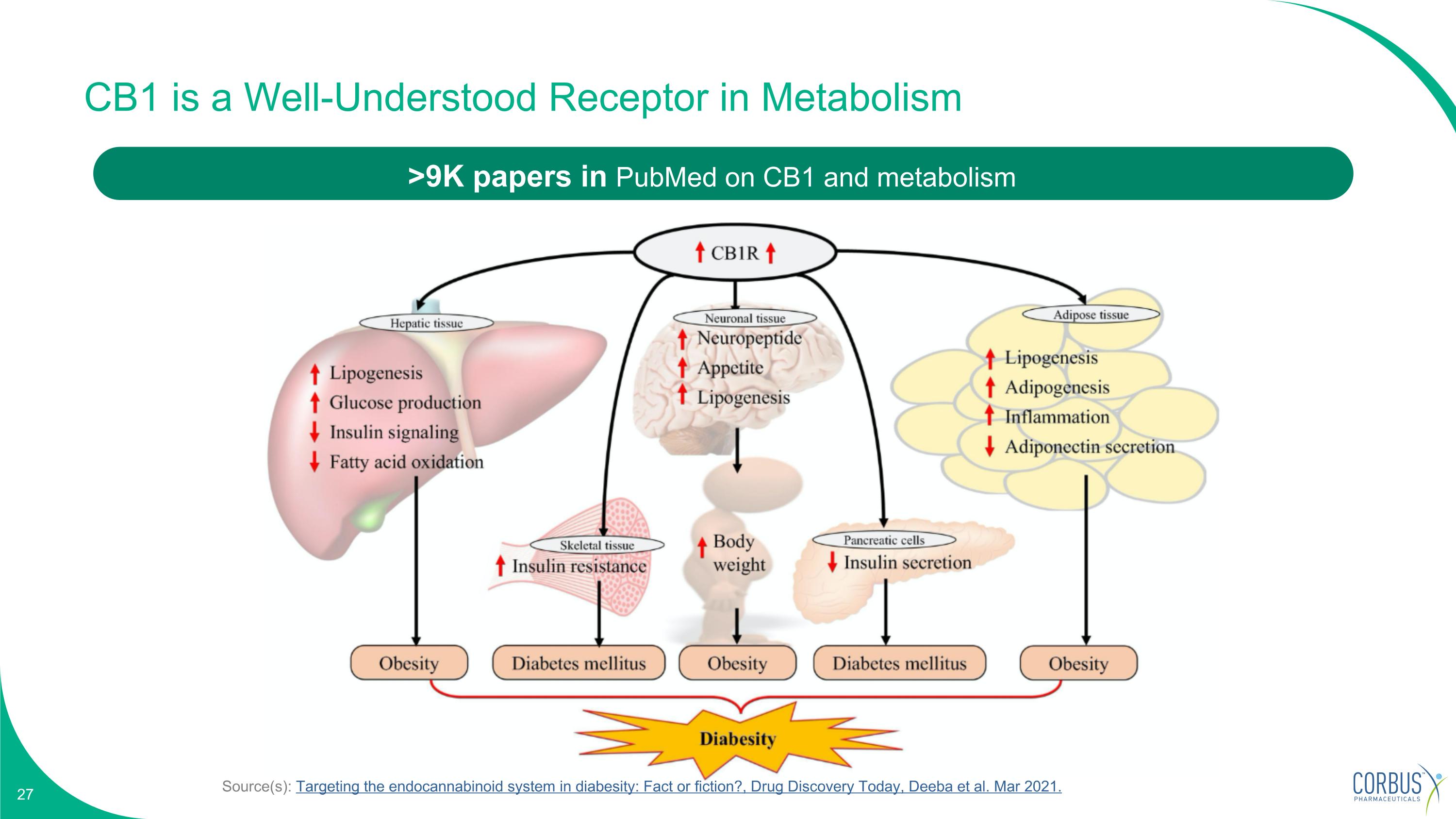
Source(s): Targeting the endocannabinoid system in diabesity: Fact or fiction?, Drug Discovery Today, Deeba et al. Mar 2021. CB1 is a Well-Understood Receptor in Metabolism >9K papers in PubMed on CB1 and metabolism
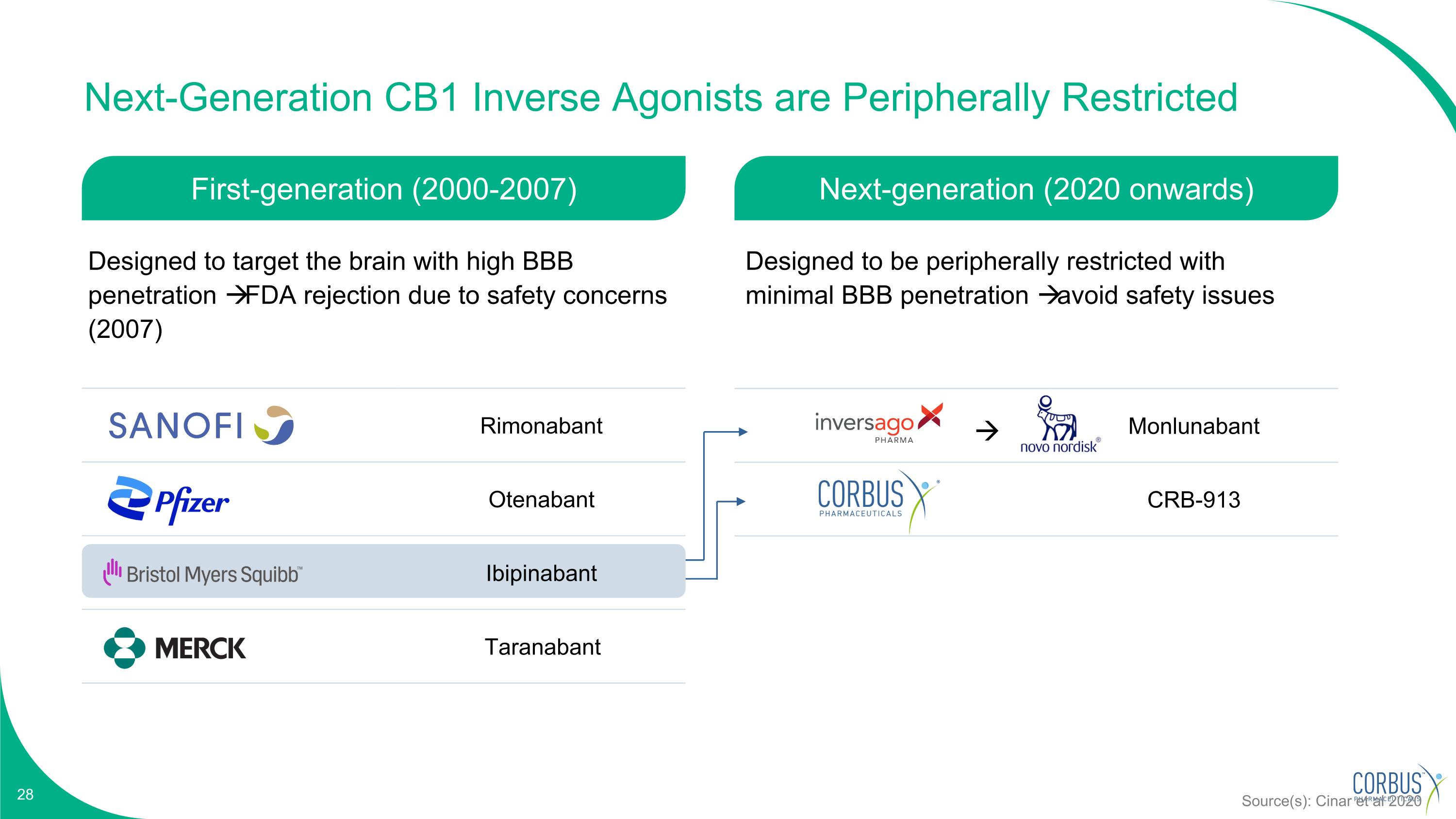
Monlunabant CRB-913 Next-Generation CB1 Inverse Agonists are Peripherally Restricted Rimonabant Otenabant Ibipinabant Taranabant Source(s): Cinar et al 2020 First-generation (2000-2007) Next-generation (2020 onwards) Designed to target the brain with high BBB penetration FDA rejection due to safety concerns (2007) Designed to be peripherally restricted with minimal BBB penetration avoid safety issues
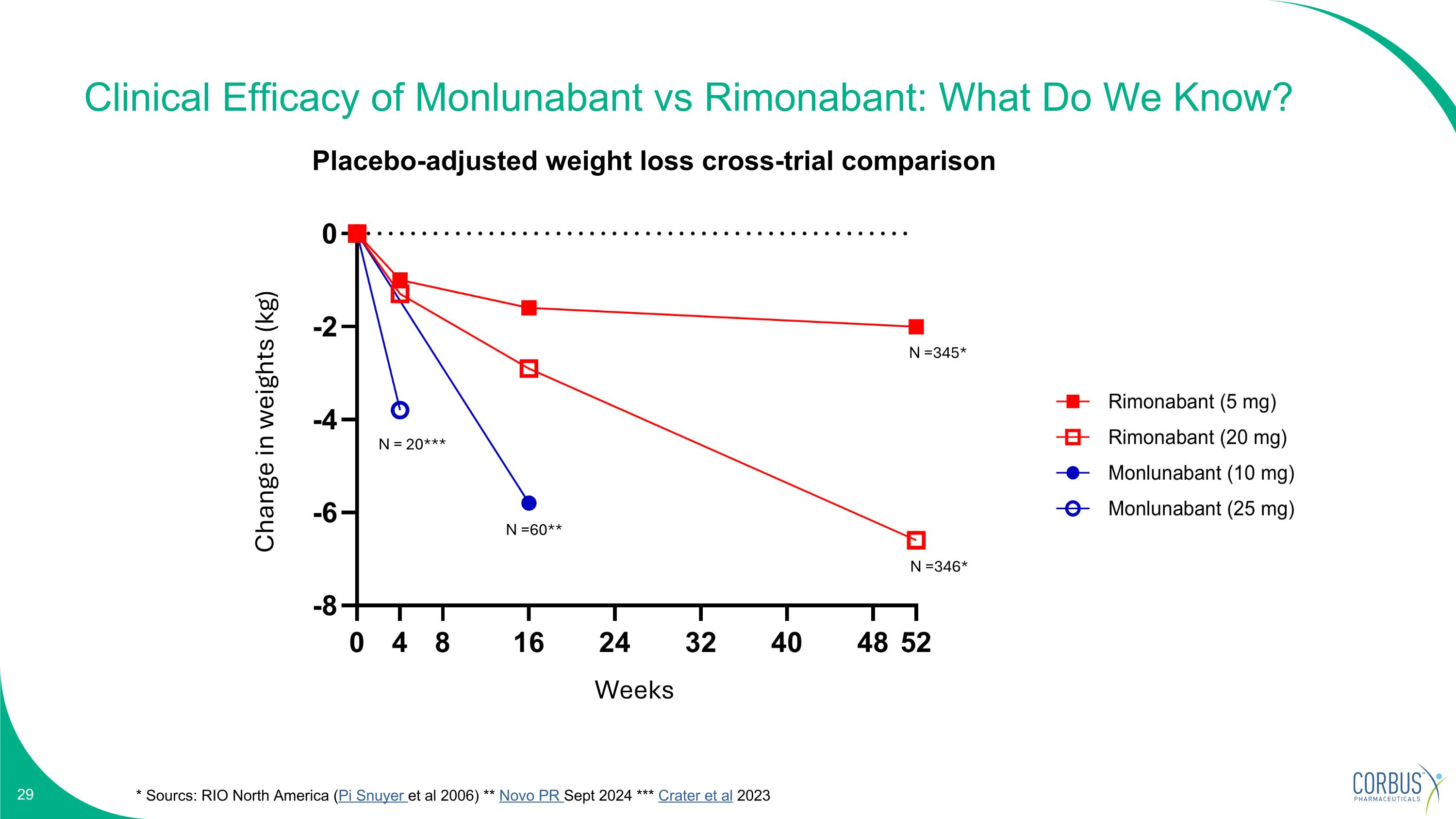
Clinical Efficacy of Monlunabant vs Rimonabant: What Do We Know? * Sourcs: RIO North America (Pi Snuyer et al 2006) ** Novo PR Sept 2024 *** Crater et al 2023 N =345* N =346* N =60** N = 20*** Placebo-adjusted weight loss cross-trial comparison Change in weights (kg) Weeks
CRB-913: Designed to be a Best-in-class Next Generation CB1 Inverse Agonist Design Goals Best-in-class peripheral restriction Protect lean mass (muscle) Retain 1st gen efficacy Enhance efficacy of incretin analogs
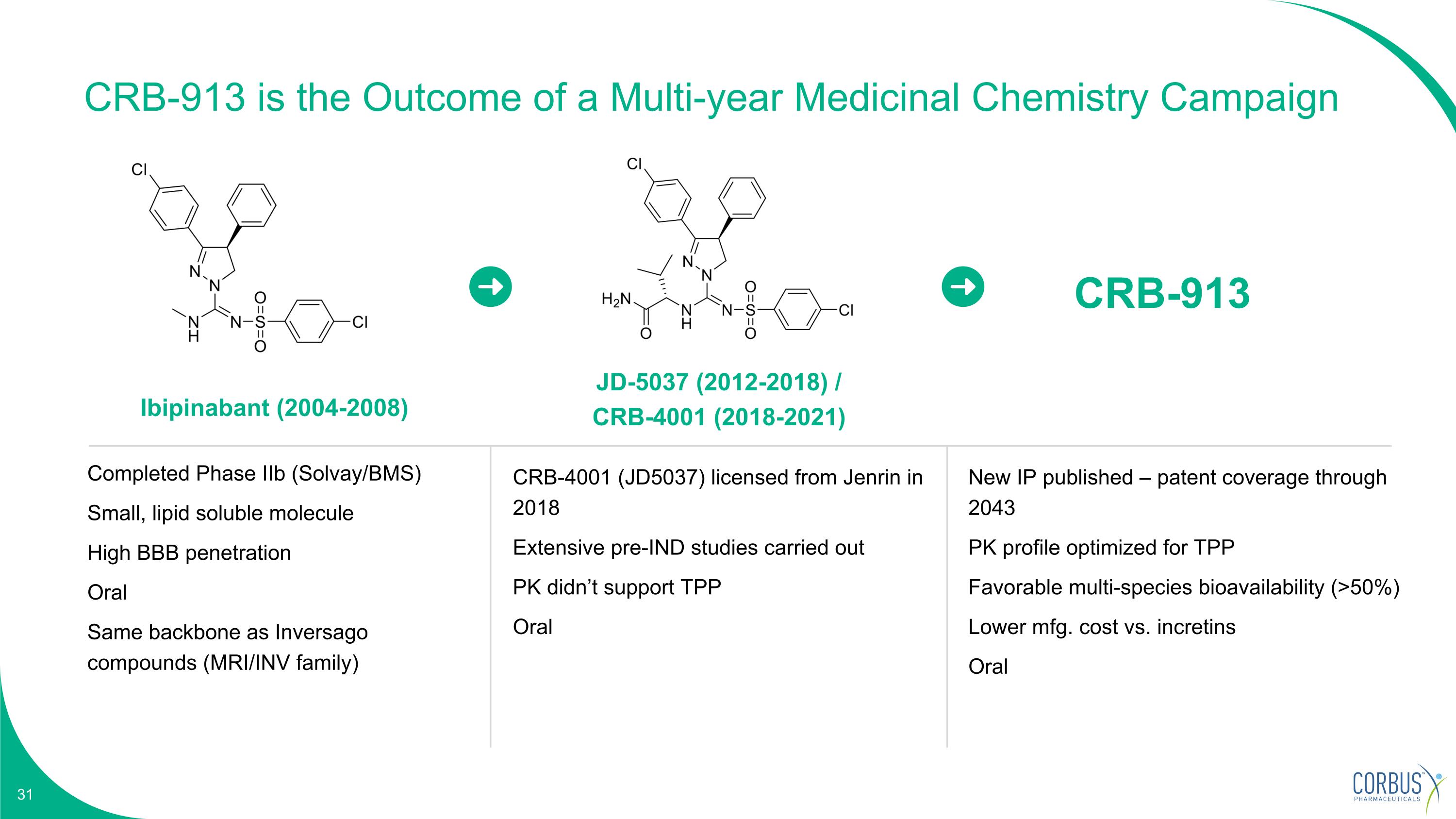
Ibipinabant (2004-2008) JD-5037 (2012-2018) / CRB-4001 (2018-2021) Completed Phase IIb (Solvay/BMS) Small, lipid soluble molecule High BBB penetration Oral Same backbone as Inversago compounds (MRI/INV family) CRB-4001 (JD5037) licensed from Jenrin in 2018 Extensive pre-IND studies carried out PK didn’t support TPP Oral CRB-913 New IP published – patent coverage through 2043 PK profile optimized for TPP Favorable multi-species bioavailability (>50%) Lower mfg. cost vs. incretins Oral CRB-913 is the Outcome of a Multi-year Medicinal Chemistry Campaign
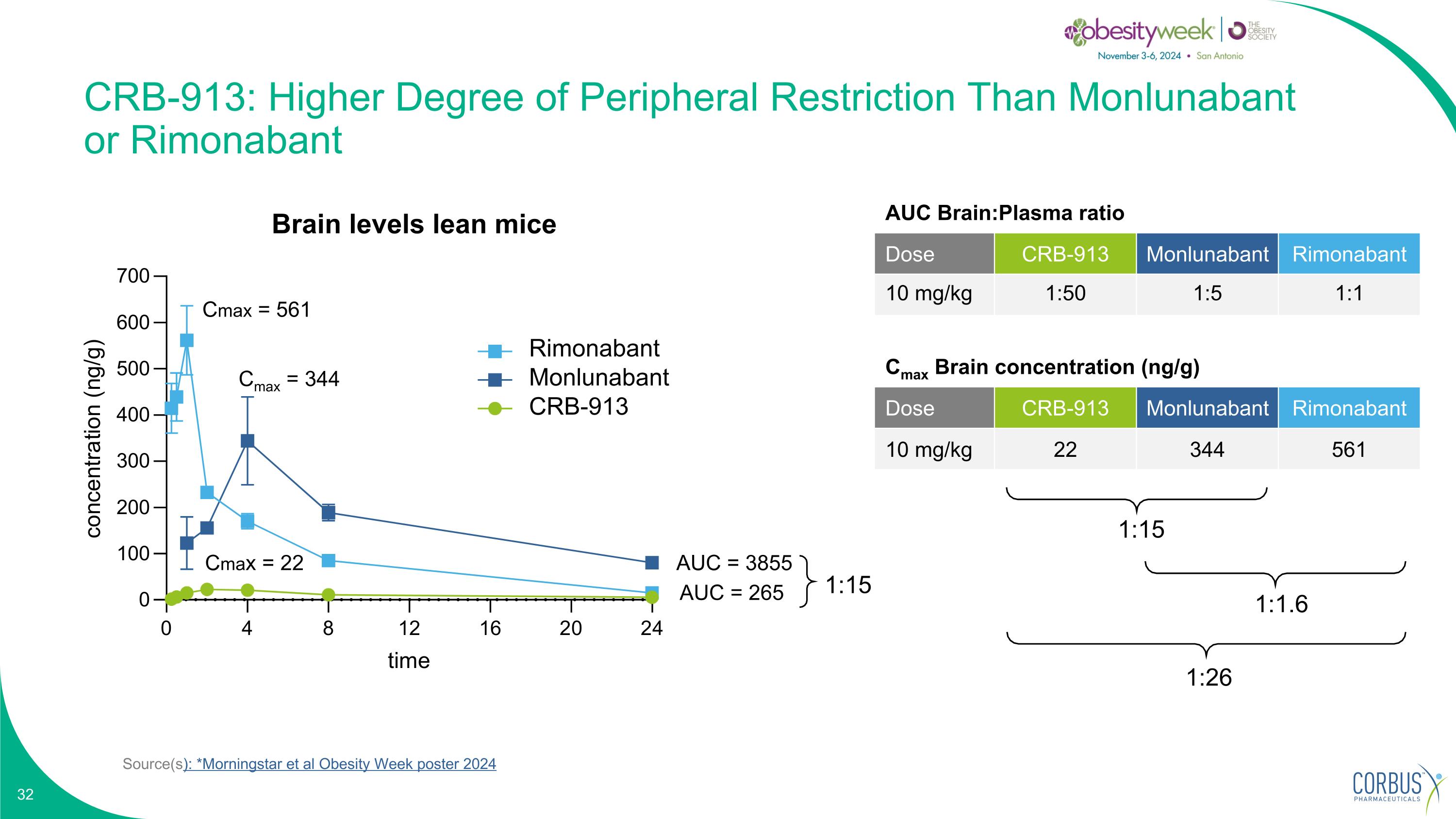
Source(s): *Morningstar et al Obesity Week poster 2024 CRB-913: Higher Degree of Peripheral Restriction Than Monlunabant or Rimonabant Cmax Brain concentration (ng/g) Dose CRB-913 Monlunabant Rimonabant 10 mg/kg 22 344 561 1:15 1:26 1:1.6 Cmax = 561 Cmax = 344 Cmax = 22 AUC = 3855 AUC = 265 1:15 Brain levels lean mice Rimonabant Monlunabant CRB-913 AUC Brain:Plasma ratio Dose CRB-913 Monlunabant Rimonabant 10 mg/kg 1:50 1:5 1:1
1. Incretin analog therapy for insensitive/intolerant/high-risk patients 2. Combination with oral incretin agonists potentially enhances efficacy OR improve tolerability 3. “Induction/maintenance” model: goal to potentially maintain weight loss post incretin analog therapy CRB-913: Potential Clinical Usage and Supportive Pre-clinical Data
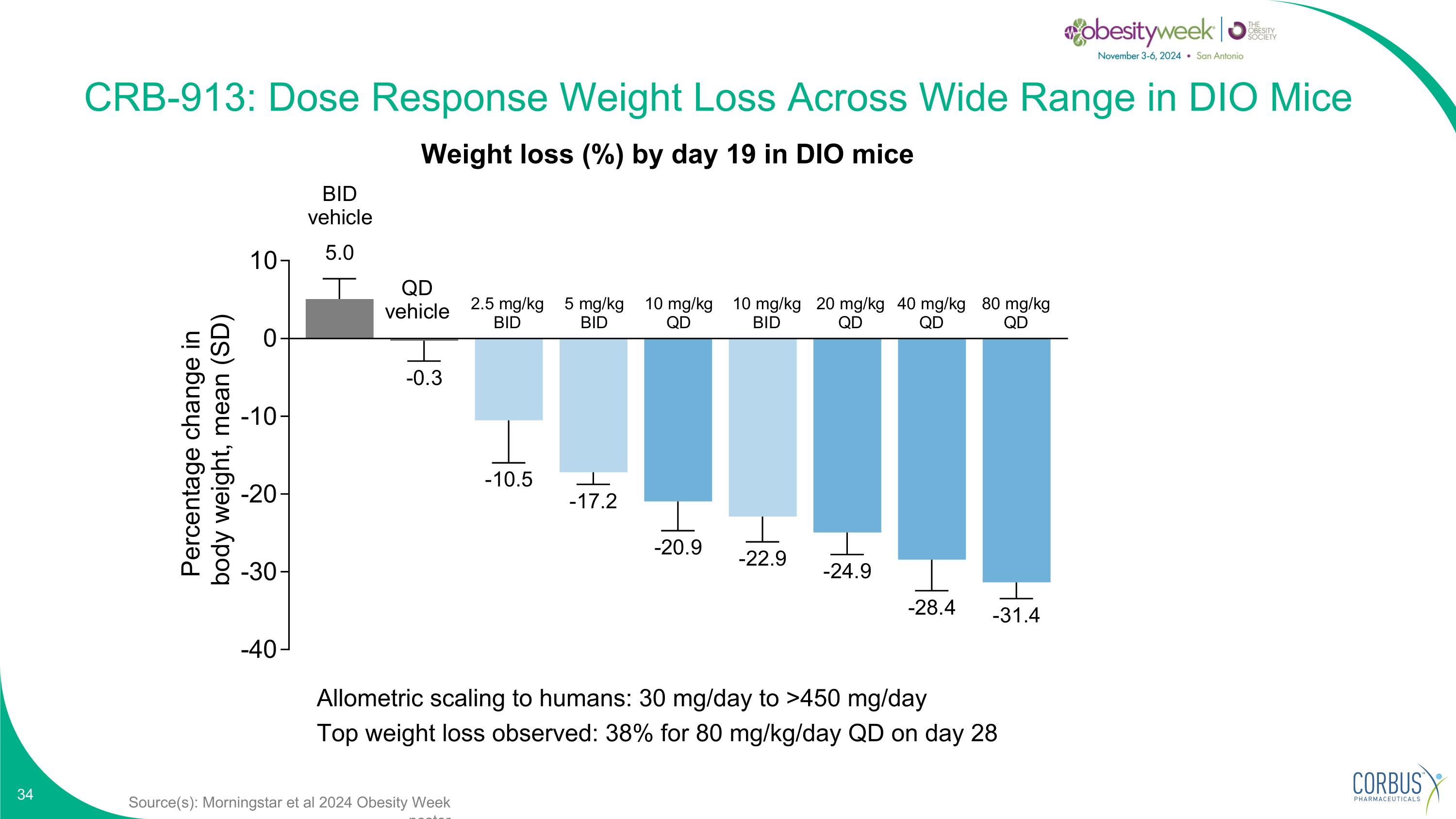
Source(s): Morningstar et al 2024 Obesity Week poster CRB-913: Dose Response Weight Loss Across Wide Range in DIO Mice Allometric scaling to humans: 30 mg/day to >450 mg/day Top weight loss observed: 38% for 80 mg/kg/day QD on day 28 Weight loss (%) by day 19 in DIO mice
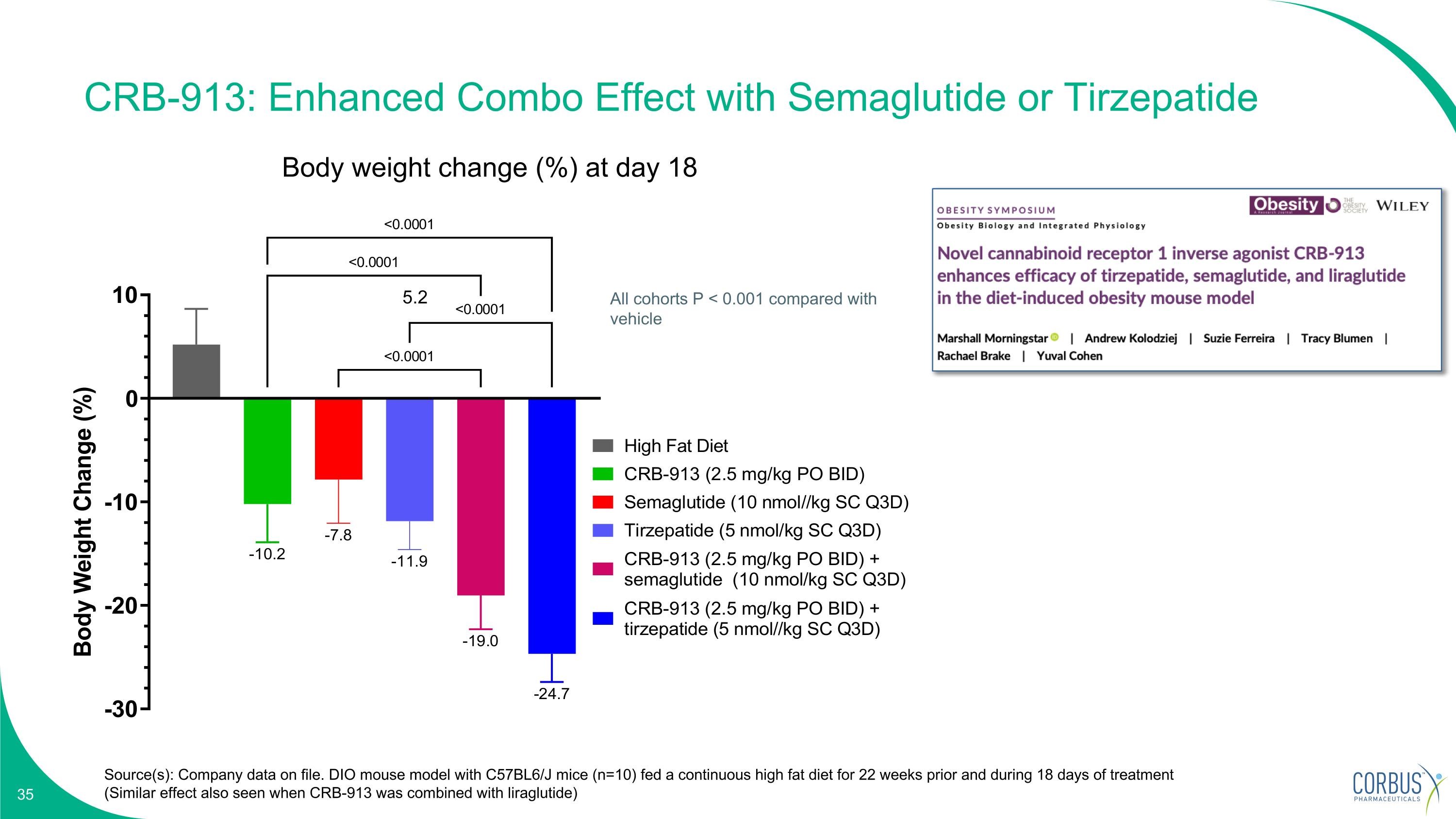
CRB-913: Enhanced Combo Effect with Semaglutide or Tirzepatide Source(s): Company data on file. DIO mouse model with C57BL6/J mice (n=10) fed a continuous high fat diet for 22 weeks prior and during 18 days of treatment (Similar effect also seen when CRB-913 was combined with liraglutide) Body weight change (%) at day 18 All cohorts P < 0.001 compared with vehicle 5.2
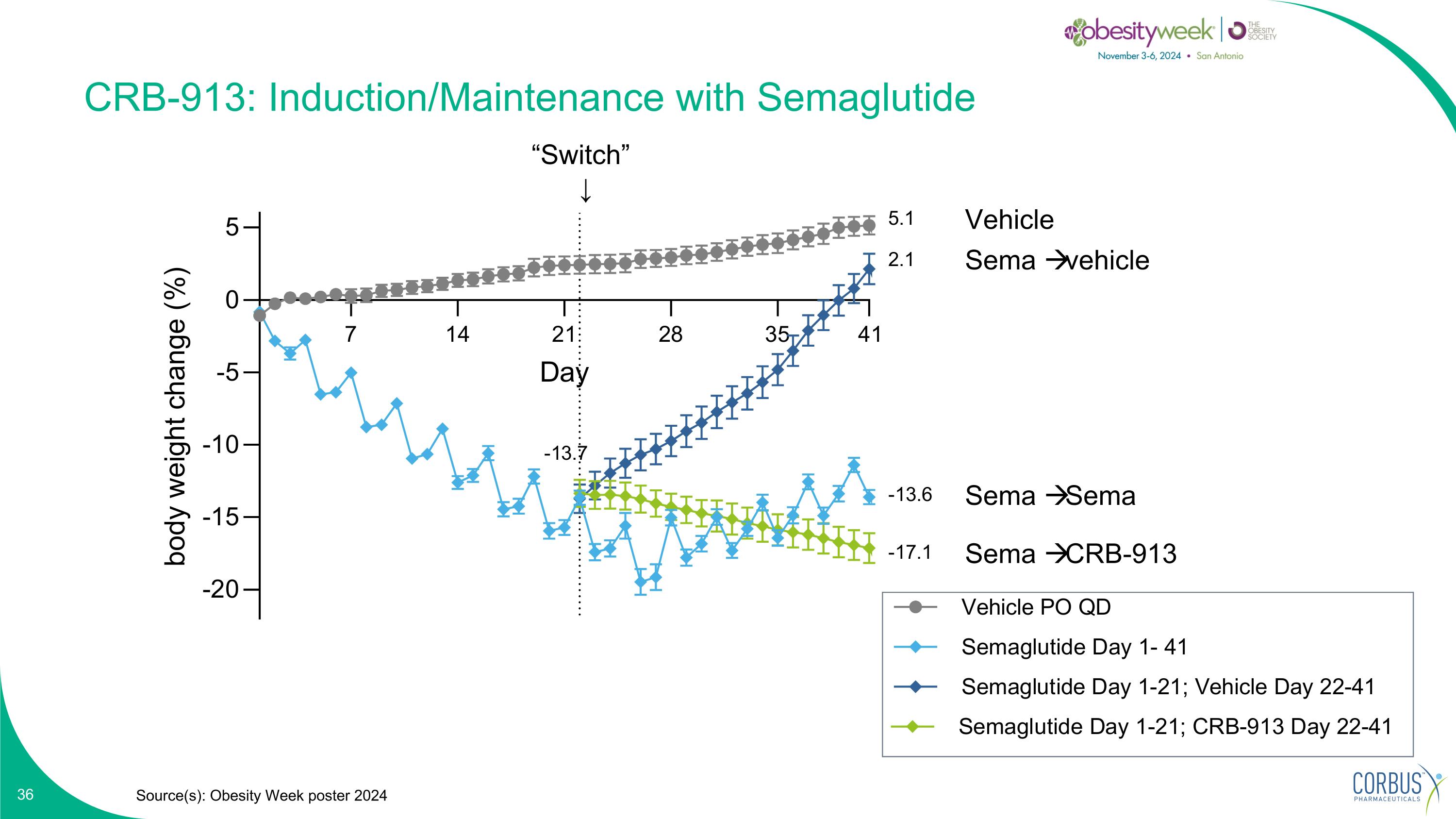
CRB-913: Induction/Maintenance with Semaglutide Source(s): Obesity Week poster 2024 “Switch” ↓ Vehicle Sema vehicle Sema Sema Sema CRB-913
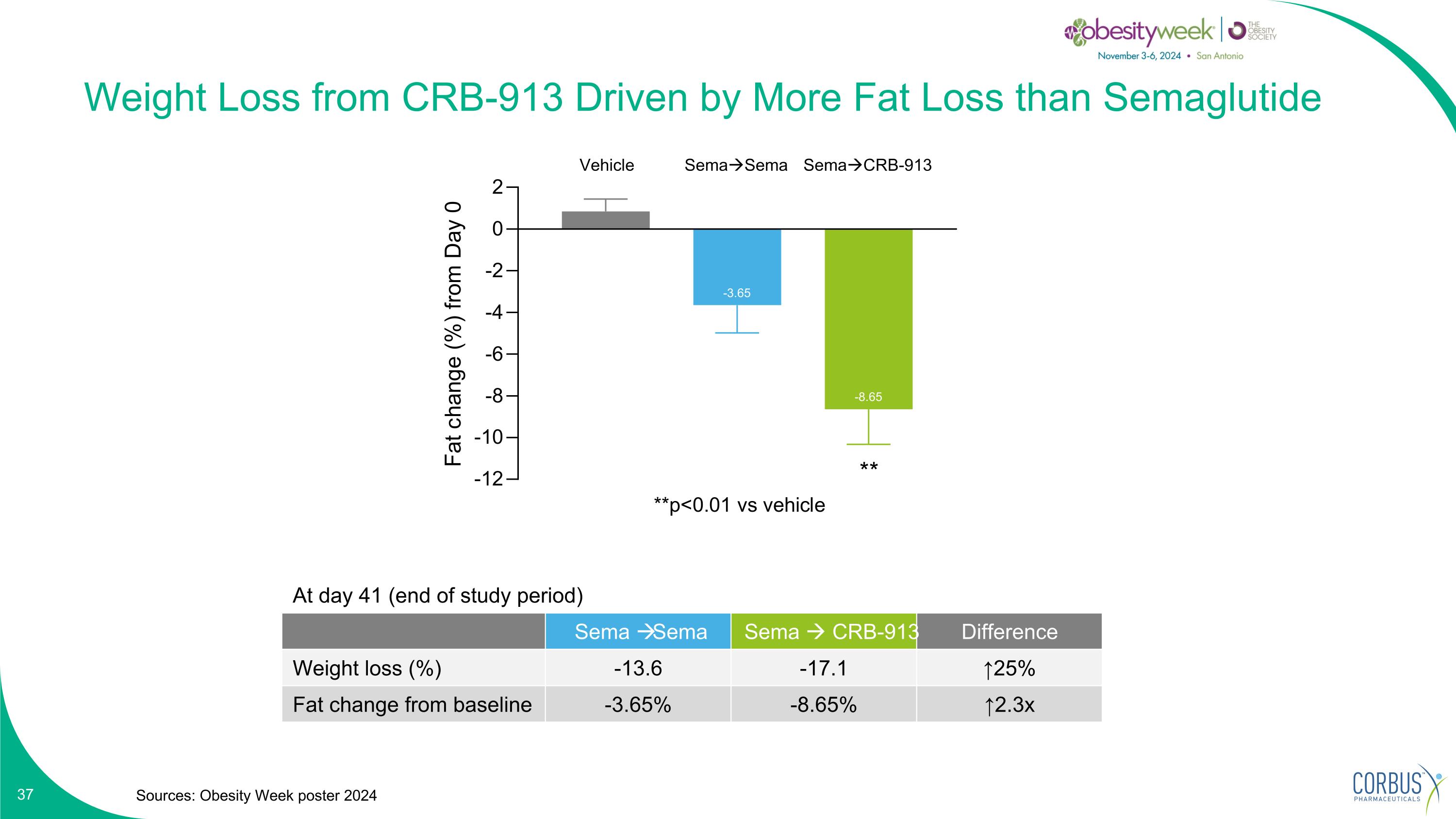
Weight Loss from CRB-913 Driven by More Fat Loss than Semaglutide Sources: Obesity Week poster 2024 SemaSema SemaCRB-913 At day 41 (end of study period) Sema Sema Sema CRB-913 Difference Weight loss (%) -13.6 -17.1 ↑25% Fat change from baseline -3.65% -8.65% ↑2.3x Vehicle
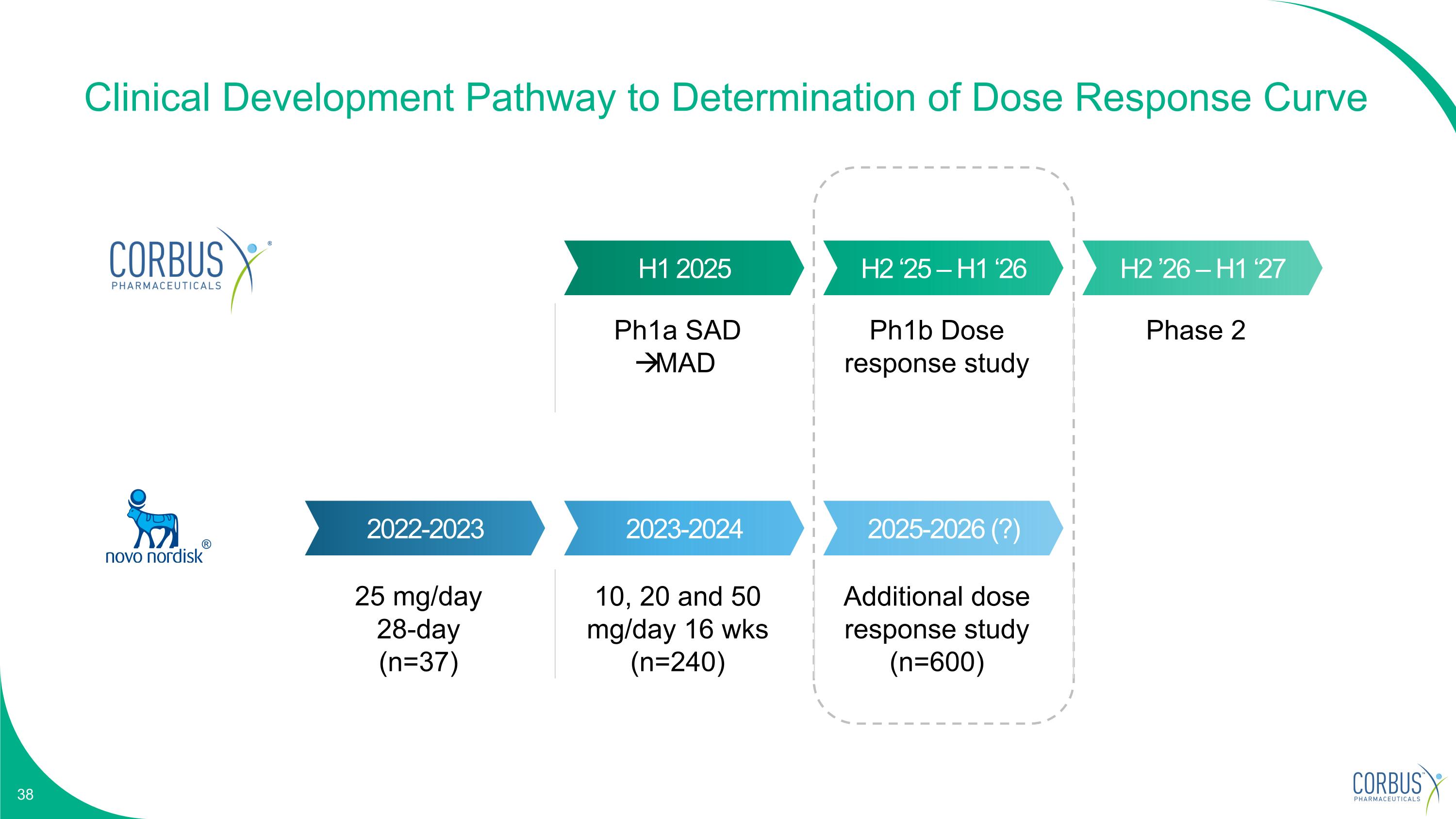
Clinical Development Pathway to Determination of Dose Response Curve H1 2025 H2 ‘25 – H1 ‘26 H2 ’26 – H1 ‘27 2022-2023 2023-2024 2025-2026 (?) 25 mg/day 28-day (n=37) 10, 20 and 50 mg/day 16 wks (n=240) Additional dose response study (n=600) Ph1a SAD MAD Ph1b Dose response study Phase 2

CRB-601 Potential “best-in-class” ⍺vβ8 mAb
CRB-601 has the Potential to Enhance Checkpoint Inhibition Focus on adopting a precision-targeted approach Novel mechanism to target TGFb in the tumor microenvironment Large opportunity potential if POC is validated
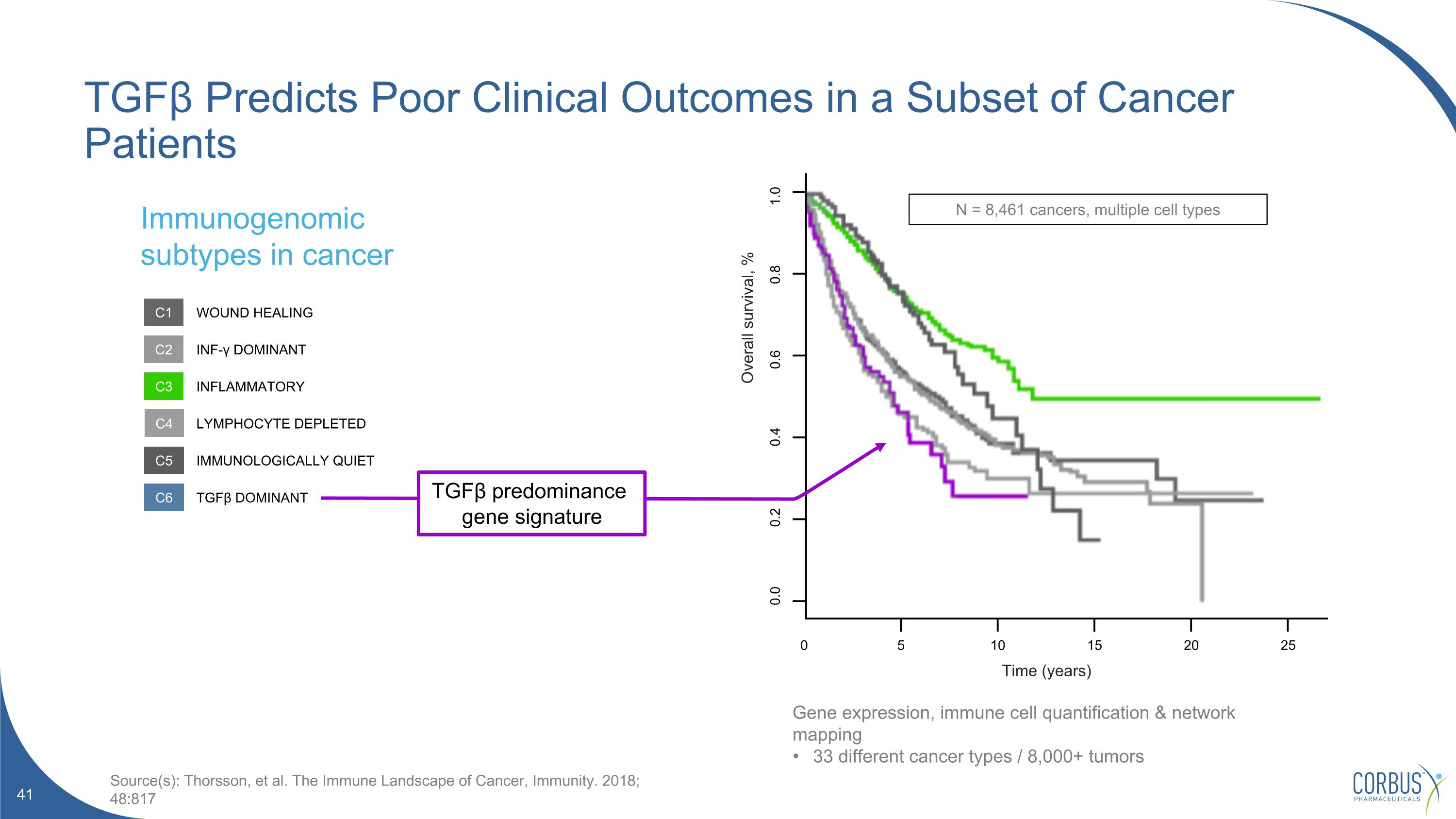
TGFβ Predicts Poor Clinical Outcomes in a Subset of Cancer Patients 0 25 20 15 10 5 0.0 0.2 0.4 0.6 0.8 1.0 N = 8,461 cancers, multiple cell types Time (years) Overall survival, % Immunogenomic subtypes in cancer Source(s): Thorsson, et al. The Immune Landscape of Cancer, Immunity. 2018; 48:817 C1 C2 C3 C4 C5 C6 WOUND HEALING INF-γ DOMINANT INFLAMMATORY LYMPHOCYTE DEPLETED IMMUNOLOGICALLY QUIET TGFβ DOMINANT TGFβ predominance gene signature Gene expression, immune cell quantification & network mapping 33 different cancer types / 8,000+ tumors
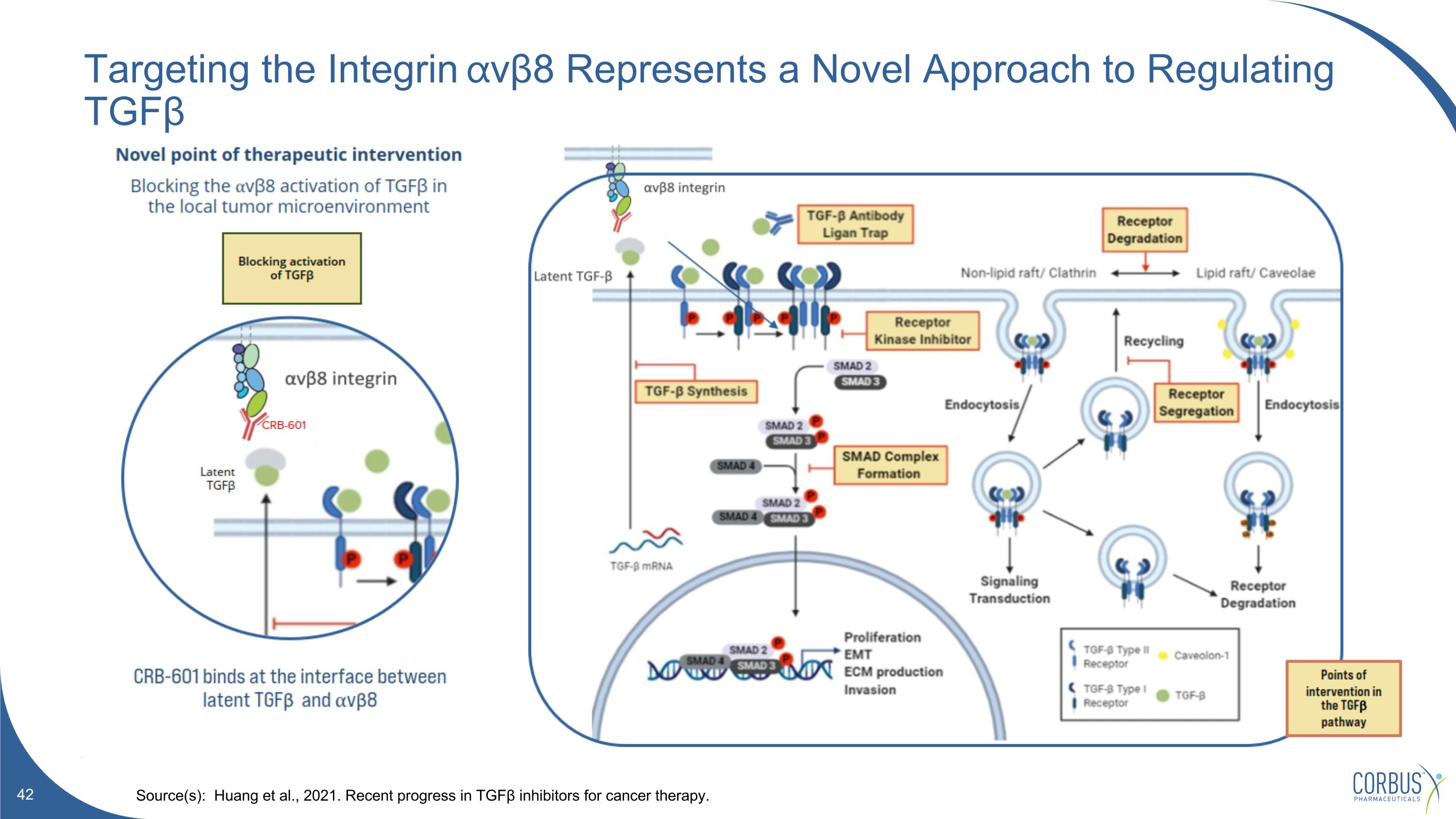
Targeting the Integrin ⍺vβ8 Represents a Novel Approach to Regulating TGFβ Source(s): Huang et al., 2021. Recent progress in TGFβ inhibitors for cancer therapy.
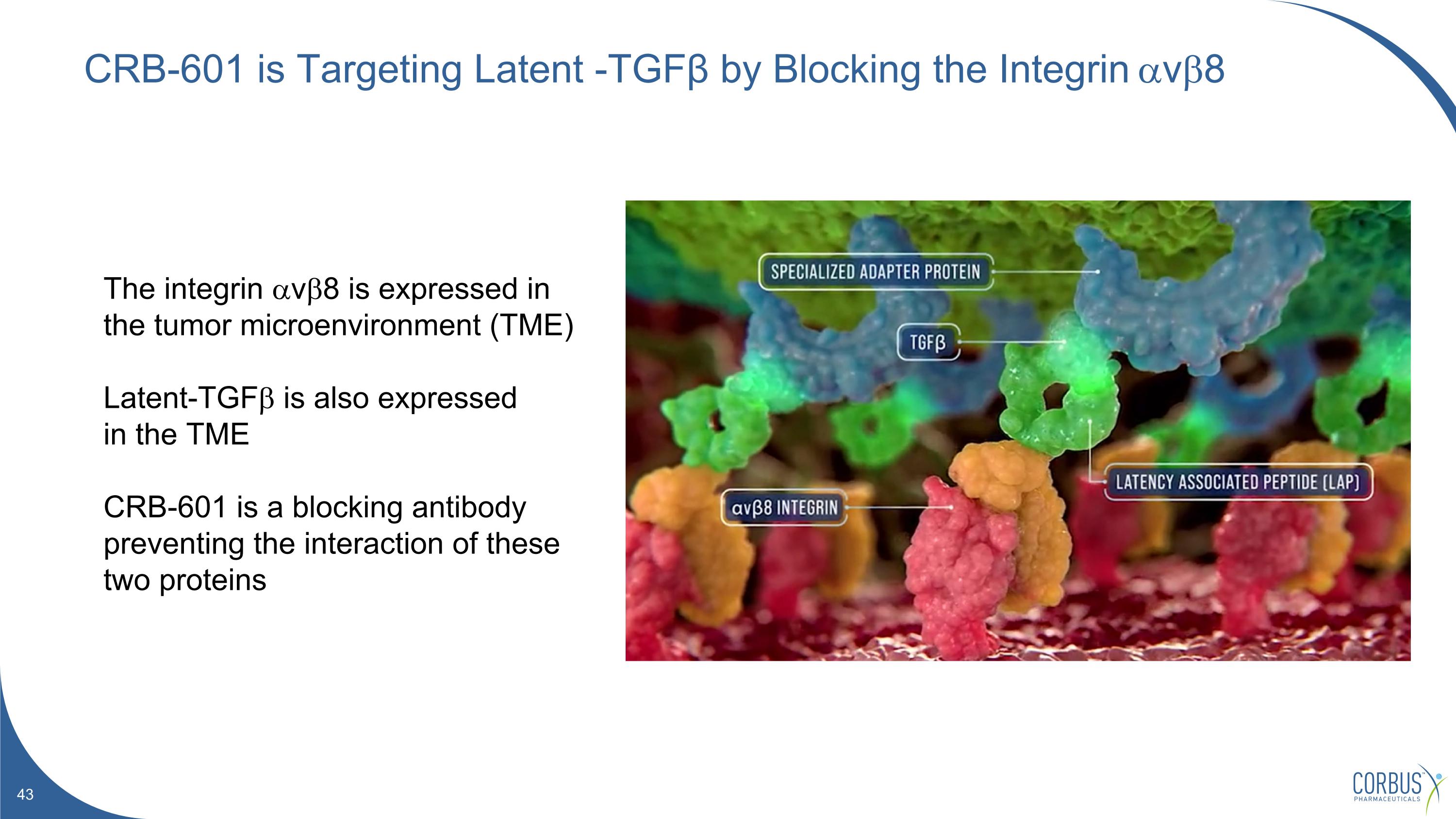
CRB-601 is Targeting Latent -TGFβ by Blocking the Integrin avb8 The integrin avb8 is expressed in the tumor microenvironment (TME) Latent-TGFb is also expressed in the TME CRB-601 is a blocking antibody preventing the interaction of these two proteins
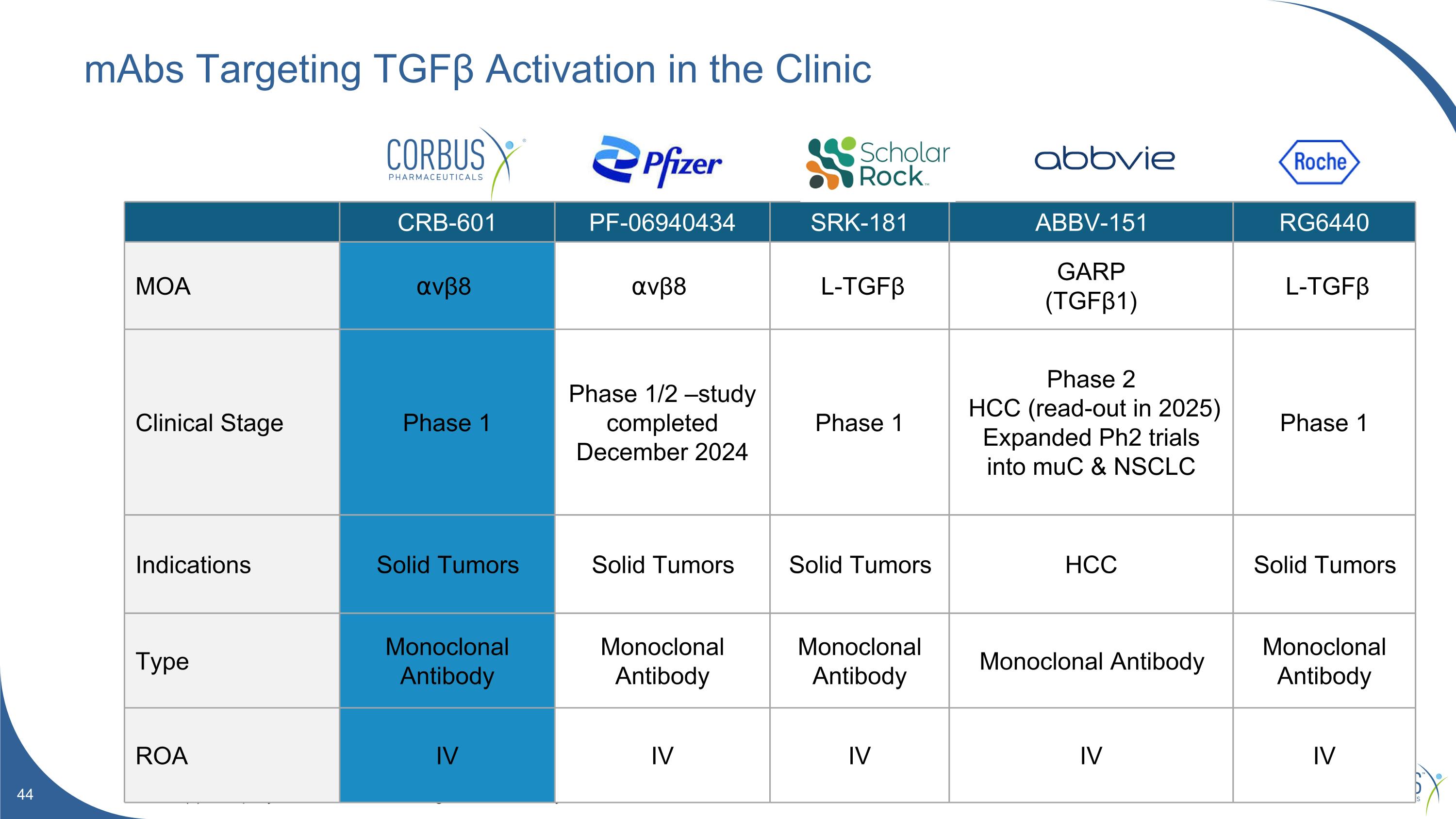
mAbs Targeting TGFβ Activation in the Clinic Source(s): Company websites. Clinicaltrials.gov. Internal analysis. CRB-601 PF-06940434 SRK-181 ABBV-151 RG6440 MOA ⍺vβ8 ⍺vβ8 L-TGFβ GARP (TGFβ1) L-TGFβ Clinical Stage Phase 1 Phase 1/2 –study completed December 2024 Phase 1 Phase 2 HCC (read-out in 2025) Expanded Ph2 trials into muC & NSCLC Phase 1 Indications Solid Tumors Solid Tumors Solid Tumors HCC Solid Tumors Type Monoclonal Antibody Monoclonal Antibody Monoclonal Antibody Monoclonal Antibody Monoclonal Antibody ROA IV IV IV IV IV
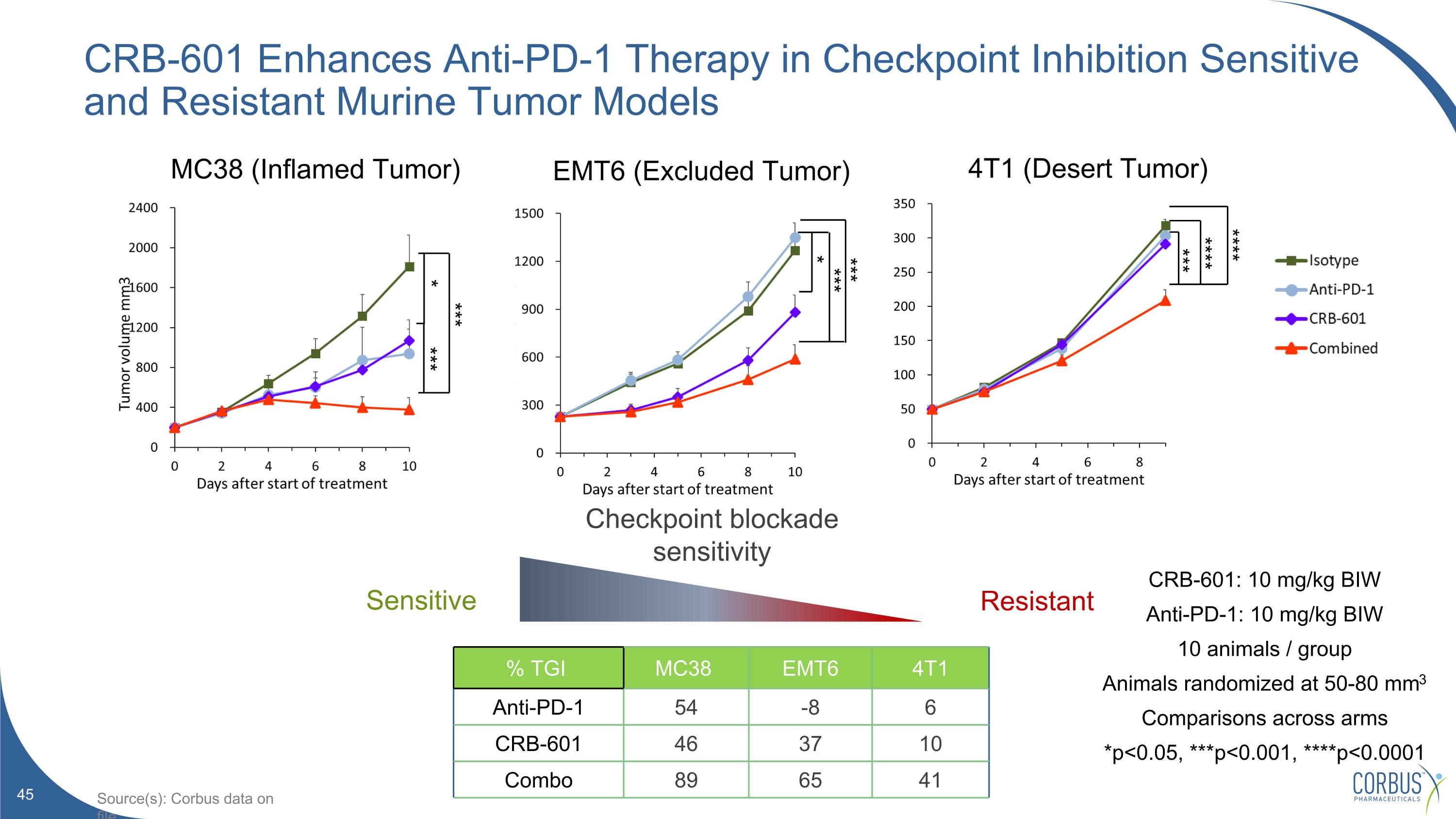
CRB-601 Enhances Anti-PD-1 Therapy in Checkpoint Inhibition Sensitive and Resistant Murine Tumor Models CRB-601: 10 mg/kg BIW Anti-PD-1: 10 mg/kg BIW 10 animals / group Animals randomized at 50-80 mm3 Comparisons across arms *p<0.05, ***p<0.001, ****p<0.0001 % TGI MC38 EMT6 4T1 Anti-PD-1 54 -8 6 CRB-601 46 37 10 Combo 89 65 41 Resistant Checkpoint blockade sensitivity Sensitive MC38 (Inflamed Tumor) EMT6 (Excluded Tumor) 4T1 (Desert Tumor) *** **** **** *** * *** *** *** * Source(s): Corbus data on file
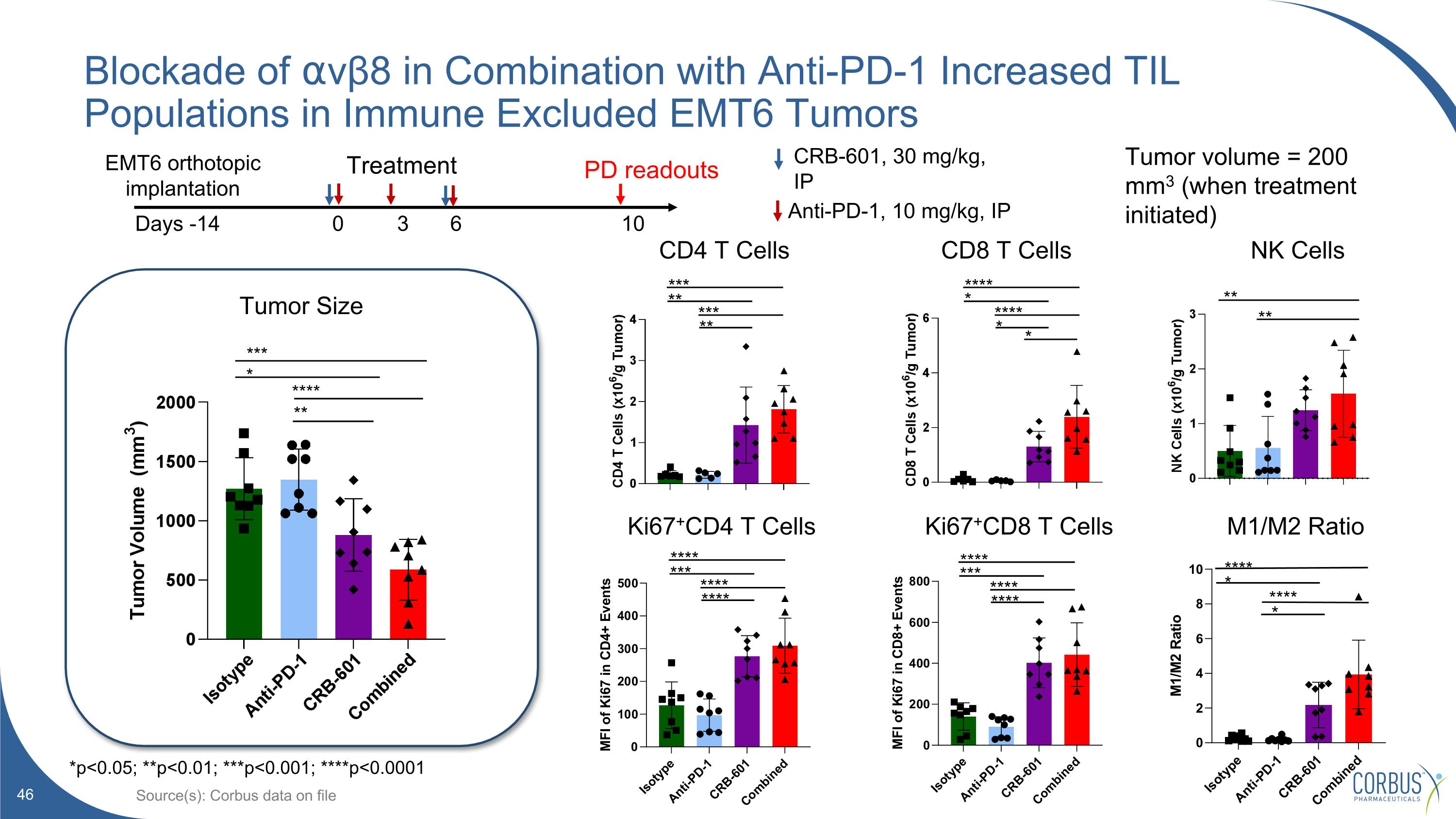
Blockade of ⍺vβ8 in Combination with Anti-PD-1 Increased TIL Populations in Immune Excluded EMT6 Tumors Source(s): Corbus data on file **** *** **** **** **** *** **** **** Ki67+CD4 T Cells Ki67+CD8 T Cells CD4 T Cells CD8 T Cells Tumor Size * *** * **** ** *** ** *** ** **** * **** * Treatment Days -14 0 3 6 10 Anti-PD-1, 10 mg/kg, IP CRB-601, 30 mg/kg, IP EMT6 orthotopic implantation PD readouts Tumor volume = 200 mm3 (when treatment initiated) *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001 M1/M2 Ratio NK Cells ** ** **** **** * *
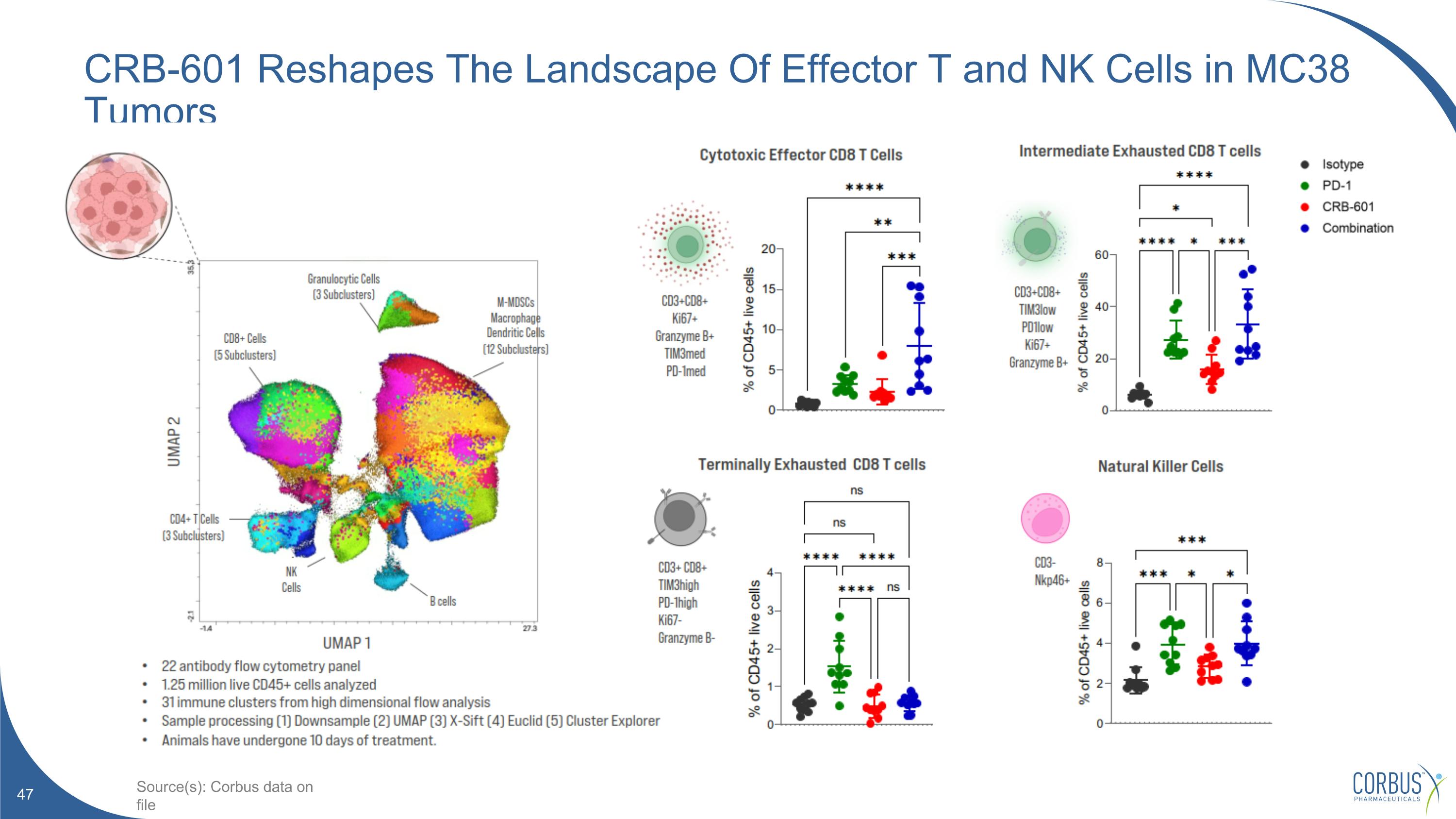
CRB-601 Reshapes The Landscape Of Effector T and NK Cells in MC38 Tumors Source(s): Corbus data on file
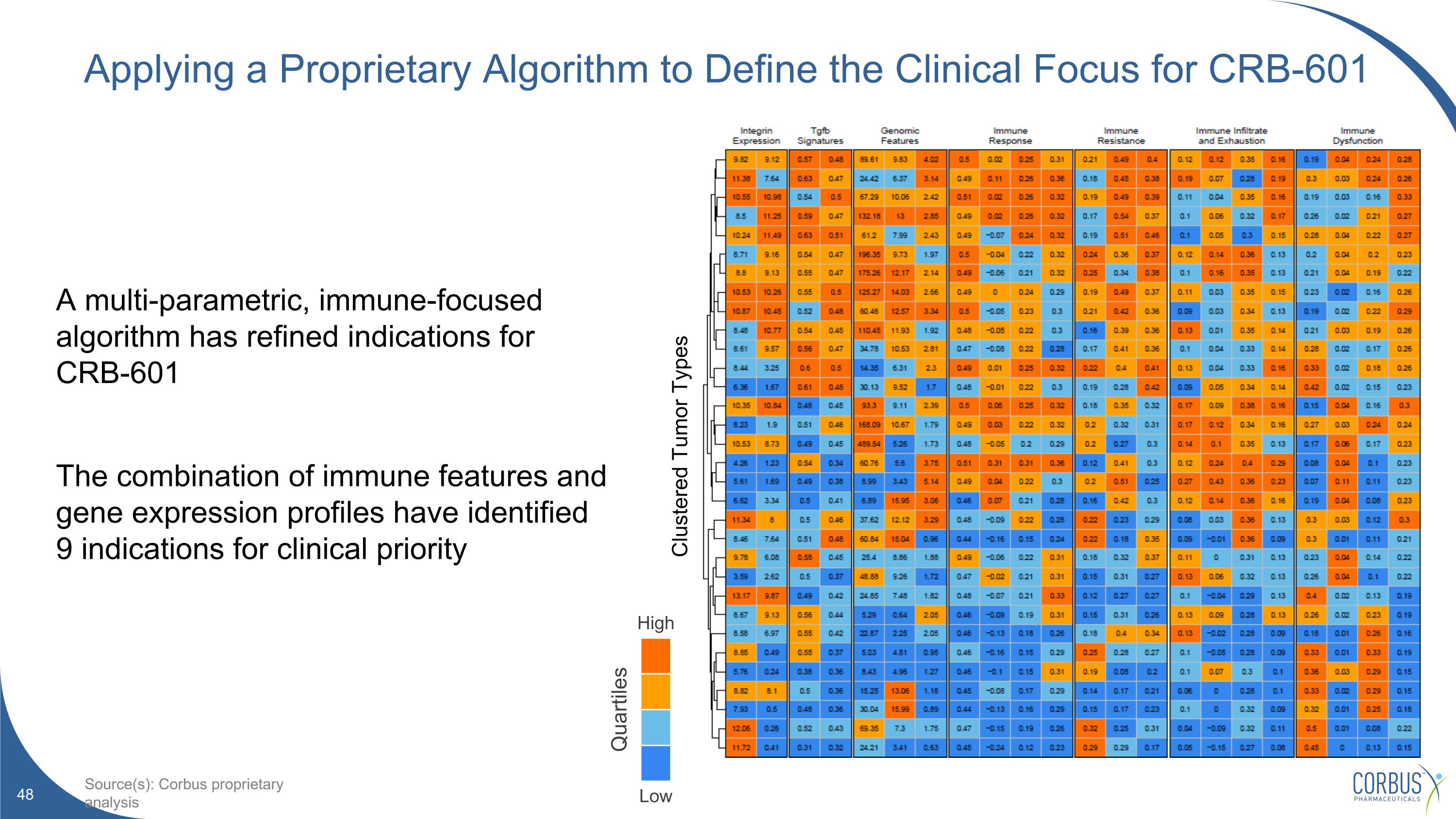
Applying a Proprietary Algorithm to Define the Clinical Focus for CRB-601 A multi-parametric, immune-focused algorithm has refined indications for CRB-601 The combination of immune features and gene expression profiles have identified 9 indications for clinical priority High Low Quartiles Source(s): Corbus proprietary analysis Clustered Tumor Types
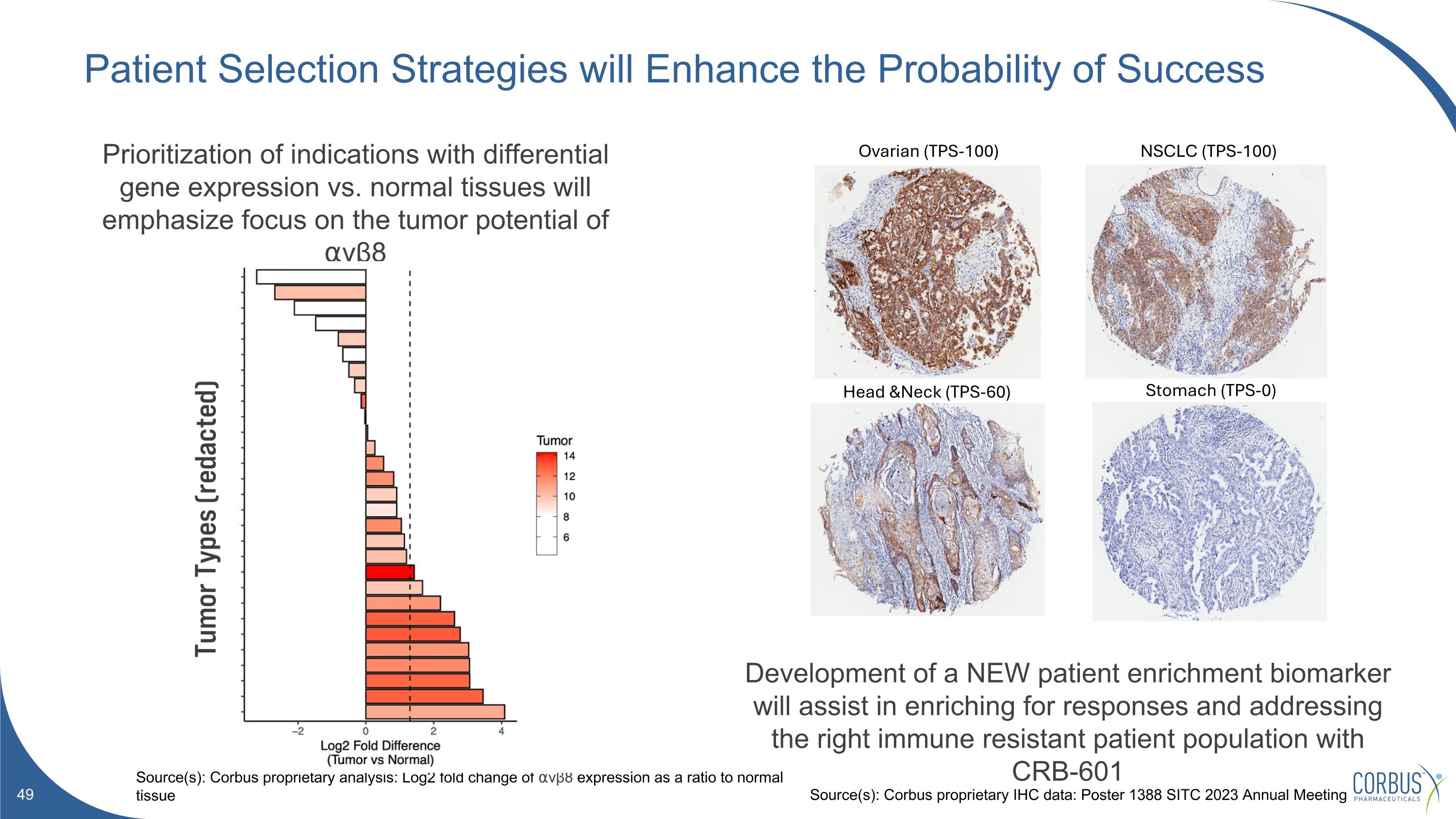
Patient Selection Strategies will Enhance the Probability of Success Source(s): Corbus proprietary analysis: Log2 fold change of ⍺vβ8 expression as a ratio to normal tissue Prioritization of indications with differential gene expression vs. normal tissues will emphasize focus on the tumor potential of ⍺vβ8 Development of a NEW patient enrichment biomarker will assist in enriching for responses and addressing the right immune resistant patient population with CRB-601 NSCLC (TPS-100) Head &Neck (TPS-60) Ovarian (TPS-100) Stomach (TPS-0) Source(s): Corbus proprietary IHC data: Poster 1388 SITC 2023 Annual Meeting

Leadership Upcoming Catalysts Financials
Management Team Sean Moran, CPA, MBA Chief Financial Officer Corbus co-founder and Chief Financial Officer since 2014. Prior senior financial management experience in emerging biotech and medical device companies. Christina Bertsch Head of Human Resources Accomplished senior human resource executive providing strategic HR consulting services to both large and small businesses across a variety of industries. Yuval Cohen, PhD Chief Executive Officer, Director Corbus co-founder and Chief Executive Officer since 2014. Previously the President and co-founder of Celsus Therapeutics from 2005. Dominic Smethurst, PhD Chief Medical Officer, MA MRCP Dr. Smethurst, MA MRCP, joined Corbus as our Chief Medical Officer in February 2024. He most recently served as CMO of Bicycle Therapeutics.

Board of Directors Amb. Alan Holmer Ret. Chairman of the Board More than two decades of public service in Washington, D.C. including Special Envoy to China; Former CEO of PhRMA. Winston Kung, MBA Director More than 20 years of senior financial, business development and investment banking experience; currently CFO of ArriVent. (NASDAQ:AVBP) Yuval Cohen, PhD Chief Executive Officer, Director Corbus co-founder and Chief Executive Officer since 2014. Previous the President and co-founder of Celsus Therapeutics from 2005. Anne Altmeyer, PhD, MBA, MPH Director 20 years of experience advancing oncology R&D programs and leading impactful corporate development transactions; currently President & CEO of TigaTx. Yong (Ben) Ben, MD, MBA Director 25 years of oncology R&D experience across industry and academia. CMO of BridgeBio Oncology Therapeutics and former CMO of BeiGene. Rachelle Jacques Director More than 25-year professional career, experience in U.S. and global biopharmaceutical commercial leadership, including multiple high-profile product launches in rare diseases; Former CEO of Akari Therapeutics. (NASDAQ: AKTX) John K. Jenkins, MD Director Distinguished 25-year career serving at the U.S. FDA, including 15 years of senior leadership in CDER and OND. Pete Salzmann, MD, MBA Director 20 years of industry experience and currently serves as Chief Executive Officer of Immunovant (NASDAQ: IMVT), a biopharmaceutical company focused on developing therapies for patients with autoimmune diseases.
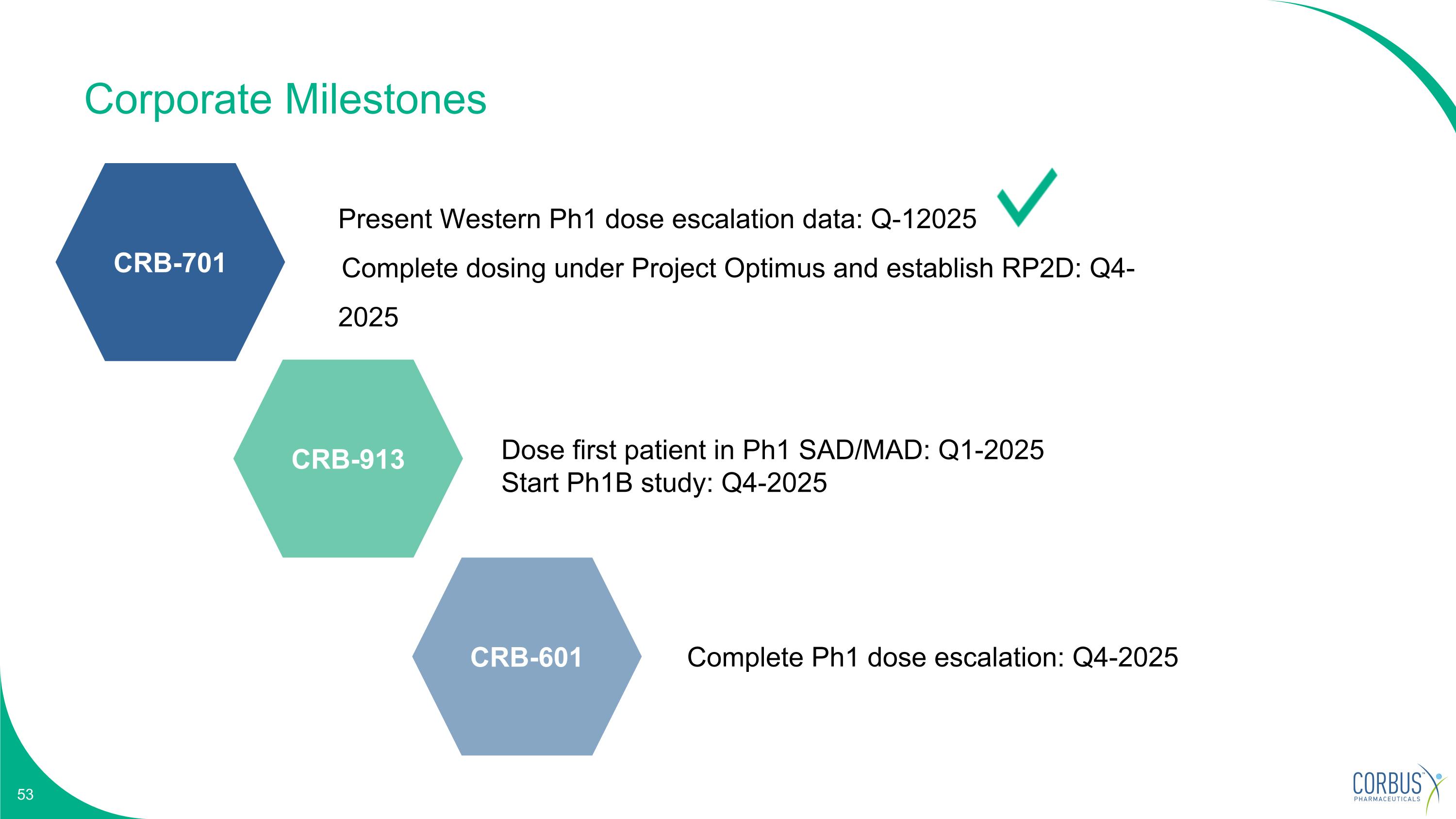
Corporate Milestones Dose first patient in Ph1 SAD/MAD: Q1-2025 Start Ph1B study: Q4-2025 Complete Ph1 dose escalation: Q4-2025 Present Western Ph1 dose escalation data: Q-12025 Complete dosing under Project Optimus and establish RP2D: Q4-2025 CRB-701 CRB-913 CRB-601
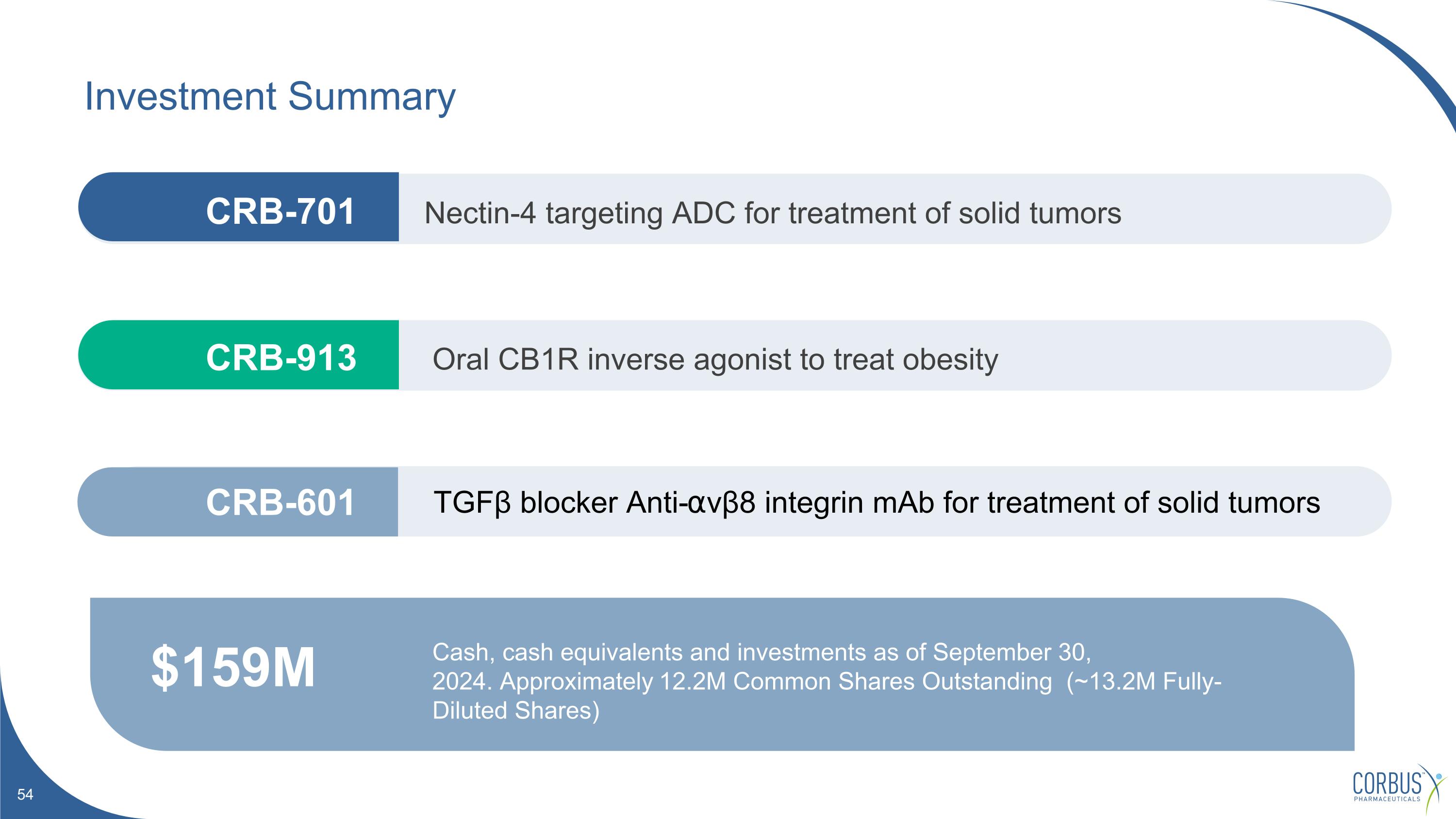
Investment Summary $159M Cash, cash equivalents and investments as of September 30, 2024. Approximately 12.2M Common Shares Outstanding (~13.2M Fully-Diluted Shares) Nectin-4 targeting ADC for treatment of solid tumors Oral CB1R inverse agonist to treat obesity TGFβ blocker Anti-⍺vβ8 integrin mAb for treatment of solid tumors CRB-913 CRB-601 CRB-701